Abstract
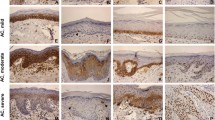
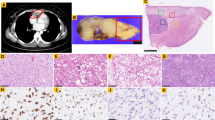
Molecular mechanisms of lip squamous cell carcinoma (LSCC) and actinic cheilitis (AC) are unclear. We aimed at assessing loss of heterozygosity (LOH) and TP53 and BRAF V600E mutations in these lesions. Formalin-fixed paraffin-embedded (FFPE) samples of 17 LSCC and 16 AC were included, with additional 5 fresh LSCC genotyped for TP53 mutations. LOH was assessed by six polymorphic markers located at 9p22, 9q22, and 17p13 and correlated with cell proliferation (Ki-67) and P53 immunostaining. Direct sequencing of TP53 exons 2–11 (fresh samples), and exons 5–9 (FFPE samples) was carried out. BRAF V600E mutation was genotyped in eight LSCC. LOH occurred in at least one marker in 15/17 LSCC and in 9/16 AC. The marker exhibiting the highest frequency of allelic loss (FAL) in LSCC was D9S157 (8/12 informative cases) and D9S287 in AC (4/11 informative cases). Cell proliferation was not correlated with LOH or with the FAL and no correlation between P53 IHC and 17p LOH was observed. We found TP53 missense mutations in both lesions and nonsense in LSCC, including CC>TT transition, which is a marker of UV damage. BRAF V600E mutation was not detected. LOH and TP53 mutations detected in LSCC and AC may be associated with tumorigenesis, whereas BRAF V600E mutation does not seem to significantly contribute to LSCC pathogenesis.


Similar content being viewed by others
References
INCA. Estimativa 2014 – incidência de câncer no Brasil. Instituto Nacional de Câncer José Alencar Gomes da Silva 2014;1:124.
Pogoda JM, Preston-Martin S. Solar radiation, lip protection, and lip cancer risk in Los Angeles County women (California, United States). Cancer Causes Control. 1996;7(4):458–63.
Souza RL, Fonseca-Fonseca T, Oliveira-Santos CC, Correa GT, Santos FB, Cardoso CM, et al. Lip squamous cell carcinoma in a Brazilian population: epidemiological study and clinicopathological associations. Med Oral Patol Oral Cir Bucal. 2011;16(6):e757–62.
Sugerman PB, Savage NW. Oral cancer in Australia: 1983–1996. Aust Dent J. 2002;47(1):45–56.
Moore S, Johnson N, Pierce A, Wilson D. The epidemiology of lip cancer: a review of global incidence and aetiology. Oral Dis. 1999;5(3):185–95.
Krolls SO, Hoffman S. Squamous cell carcinoma of the oral soft tissues: a statistical analysis of 14,253 cases by age, sex, and race of patients. J Am Dent Assoc. 1976;92(3):571–4.
Lindqvist C. Risk factors in lip cancer: a questionnaire survey. Am J Epidemiol. 1979;109(5):521–30.
Ostman J, Anneroth G, Gustafsson H, Tavelin B. Malignant oral tumours in Sweden 1960–1989—an epidemiological study. Eur J Cancer B Oral Oncol. 1995;31b(2):106–12.
Main JH, Pavone M. Actinic cheilitis and carcinoma of the lip. J Can Dent Assoc. 1994;60(2):113–6.
Kaugars GE, Pillion T, Svirsky JA, Page DG, Burns JC, Abbey LM. Actinic cheilitis: a review of 152 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(2):181–6.
Cavalcante AS, Anbinder AL, Carvalho YR. Actinic cheilitis: clinical and histological features. J Oral Maxillofac Surg. 2008;66(3):498–503. doi:10.1016/j.joms.2006.09.016.
Markopoulos A, Albanidou-Farmaki E, Kayavis I. Actinic cheilitis: clinical and pathologic characteristics in 65 cases. Oral Dis. 2004;10(4):212–6. doi:10.1111/j.1601-0825.2004.01004.x.
van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009;45(4–5):317–23. doi:10.1016/j.oraloncology.2008.05.016.
Anwar J, Wrone DA, Kimyai-Asadi A, Alam M. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol. 2004;22(3):189–96. doi:10.1016/j.clindermatol.2003.12.006.
Milasin J, Pujic N, Dedovic N, Nikolic Z, Petrovic V, Dimitrijevic B. High incidence of H-ras oncogene mutations in squamous cell carcinoma of lip vermilion. J Oral Pathol Med. 1994;23(7):298–301.
Chou A, Dekker N, Jordan RC. Identification of novel fibroblast growth factor receptor 3 gene mutations in actinic cheilitis and squamous cell carcinoma of the lip. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(4):535–41. doi:10.1016/j.tripleo.2008.12.050.
Ostwald C, Gogacz P, Hillmann T, Schweder J, Gundlach K, Kundt G, et al. p53 mutational spectra are different between squamous-cell carcinomas of the lip and the oral cavity. Int J Cancer. 2000;88(1):82–6.
Gomes CC, Fonseca-Silva T, Galvao CF, Friedman E, De Marco L, Gomez RS. Inter- and intra-lesional molecular heterogeneity of oral leukoplakia. Oral Oncol. 2015;51(2):178–81. doi:10.1016/j.oraloncology.2014.11.003.
Murali A, Sailasree R, Sebastian P, Rejnish Kumar R, Varghese BT, Kannan S. Loss of heterozygosity of D9S162: molecular predictor for treatment response in oral carcinoma. Oral Oncol. 2011;47(7):571–6. doi:10.1016/j.oraloncology.2011.04.009.
Graveland AP, Golusinski PJ, Buijze M, Douma R, Sons N, Kuik DJ, et al. Loss of heterozygosity at 9p and p53 immunopositivity in surgical margins predict local relapse in head and neck squamous cell carcinoma. Int J Cancer. 2011;128(8):1852–9. doi:10.1002/ijc.25523.
Cabral LS, Festa Neto C, Sanches Jr JA, Ruiz IR. Genomic instability in human actinic keratosis and squamous cell carcinoma. Clinics (Sao Paulo). 2011;66(4):523–8.
Saridaki Z, Liloglou T, Zafiropoulos A, Koumantaki E, Zoras O, Spandidos DA. Mutational analysis of CDKN2A genes in patients with squamous cell carcinoma of the skin. Br J Dermatol. 2003;148(4):638–48.
Kushida Y, Miki H, Ohmori M. Loss of heterozygosity in actinic keratosis, squamous cell carcinoma and sun-exposed normal-appearing skin in Japanese: difference between Japanese and Caucasians. Cancer Lett. 1999;140(1–2):169–75.
Happle R. Loss of heterozygosity in human skin. J Am Acad Dermatol. 1999;41(2 Pt 1):143–64.
Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Wiley; 2009.
Stokes A, Drozdov I, Guerra E, Ouzounis CA, Warnakulasuriya S, Gleeson MJ, et al. Copy number and loss of heterozygosity detected by SNP array of formalin-fixed tissues using whole-genome amplification. PLoS ONE. 2011;6(9):e24503. doi:10.1371/journal.pone.0024503.
Gomes CC, Fonseca-Silva T, Gomez RS. Evidence for loss of heterozygosity (LOH) at chromosomes 9p and 17p in oral granular cell tumors: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(2):249–53. doi:10.1016/j.oooo.2012.11.011.
Farias LC, Gomes CC, Brito JA, Galvao CF, Diniz MG, de Castro WH, et al. Loss of heterozygosity of the PTCH gene in ameloblastoma. Hum Pathol. 2012;43(8):1229–33. doi:10.1016/j.humpath.2011.08.026.
van Houten VM, Tabor MP, van den Brekel MW, Denkers F, Wishaupt RG, Kummer JA, et al. Molecular assays for the diagnosis of minimal residual head-and-neck cancer: methods, reliability, pitfalls, and solutions. Clin Cancer Res. 2000;6(10):3803–16.
Ribeiro GR, Francisco G, Teixeira LV, Romao-Correia RF, Sanches Jr JA, Neto CF, et al. Repetitive DNA alterations in human skin cancers. J Dermatol Sci. 2004;36(2):79–86. doi:10.1016/j.jdermsci.2004.08.003.
Ziegler A, Jonason A, Simon J, Leffell D, Brash DE. Tumor suppressor gene mutations and photocarcinogenesis. Photochem Photobiol. 1996;63(4):432–5.
Lincoln EA. Sun-induced skin changes. Prim Care. 2000;27(2):435–45.
Sinha RP, Hader DP. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. 2002;1(4):225–36.
Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28(6):622–9. doi:10.1002/humu.20495.
Wong SQ, Li J, Tan AY, Vedururu R, Pang JM, Do H, et al. Sequence artefacts in a prospective series of formalin-fixed tumours tested for mutations in hotspot regions by massively parallel sequencing. BMC Med Genomics. 2014;7:23. doi:10.1186/1755-8794-7-23.
Nakazawa H, English D, Randell PL, Nakazawa K, Martel N, Armstrong BK, et al. UV and skin cancer: specific p53 gene mutation in normal skin as a biologically relevant exposure measurement. Proc Natl Acad Sci U S A. 1994;91(1):360–4.
Weinhold N, Jacobsen A, Schultz N, Sander C, Lee W. Genome-wide analysis of noncoding regulatory mutations in cancer. Nat Genet. 2014;46(11):1160–5. doi:10.1038/ng.3101.
Supek F, Miñana B, Valcárcel J, Gabaldón T, Lehner B. Synonymous mutations frequently act as driver mutations in human cancers. Cell. 2014;156(6):1324–35. doi:10.1016/j.cell.2014.01.051.
Pracht M, Mogha A, Lespagnol A, Fautrel A, Mouchet N, Le Gall F, et al. Prognostic and predictive values of oncogenic BRAF, NRAS, c-KIT and MITF in cutaneous and mucous melanoma. J Eur Acad Dermatol Venereol. 2015. doi:10.1111/jdv.12910.
Lee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol. 2011;164(4):776–84. doi:10.1111/j.1365-2133.2010.10185.x.
Tan DS, Wang W, Leong HS, Sew PH, Lau DP, Chong FT, et al. Tongue carcinoma infrequently harbor common actionable genetic alterations. BMC Cancer. 2014;14:679. doi:10.1186/1471-2407-14-679.
Koumaki D, Kostakis G, Koumaki V, Papadogeorgakis N, Makris M, Katoulis A, et al. Novel mutations of the HRAS gene and absence of hotspot mutations of the BRAF genes in oral squamous cell carcinoma in a Greek population. Oncol Rep. 2012;27(5):1555–60. doi:10.3892/or.2012.1653.
Oberholzer PA, Kee D, Dziunycz P, Sucker A, Kamsukom N, Jones R, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012;30(3):316–21. doi:10.1200/jco.2011.36.7680.
Zaravinos A, Kanellou P, Baritaki S, Bonavida B, Spandidos DA. BRAF and RKIP are significantly decreased in cutaneous squamous cell carcinoma. Cell Cycle. 2009;8(9):1402–8.
Acknowledgments
This study was supported in part by the following Brazilian funding agencies: Coordination for the Improvement of Higher Education Personnel (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and National Council for Scientific and Technological Development (CNPq), Brazil. R.S. Gomez, C.C. Gomes, De-Paula A.M.B., and R.P. Souza are research fellows at CNPq. The authors acknowledge the Centro de Aquisição e Processamento de Imagens (CAPI- ICB/UFMG) for the LMD technical support.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Correa, G.T.B., Bernardes, V.F., de Sousa, S.F. et al. Lip cancer and pre-cancerous lesions harbor TP53 mutations, exhibit allelic loss at 9p, 9q, and 17p, but no BRAFV600E mutations. Tumor Biol. 36, 9059–9066 (2015). https://doi.org/10.1007/s13277-015-3659-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3659-9