Abstract
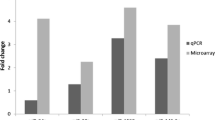
Kaposi’s sarcoma (KS) is a multicentric angioproliferative tumor of mesenchymal origin. The molecular and biologic aspects of KS are not fully understood. MicroRNAs are non-protein-coding small RNAs in the size range 19–25 nucleotides (nt) that play important roles in biological processes, including cellular differentiation, proliferation, and death. We performed a miRNA microarray analysis by detecting six paired KS and matched adjacent healthy tissues using the 7th generation of miRCURYTM LNA Array (v.18.0) (Exiqon) containing 3100 capture probes. We selected 10 significant differentially expressed miRNAs, which were confirmed by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) in 18 paired KS and matched adjacent healthy tissue specimens. We also investigated the associations between clinical features and miRNA expression. Among the 3100 human miRNA probes in the microarrays, we identified 170 differentially expressed miRNAs (69 upregulated and 101 downregulated miRNAs) in KS versus adjacent healthy tissues. Among the most significantly upregulated miRNAs were miR-126-3p, miR-199a-3p, miR-16-5p, and the 13 KSHV-related miRNAs. The most significantly downregulated miRNAs included miR-125b-1-3p and miR-1183. Eight upregulated miRNAs, miR-181b-5p, miR-199a-3p, miR-15a-5p, miR-126-3p, miR-1297, kshv-miR-k12-12-3p, kshv-miR-k12-1-5p, and miR-16-5p, and two downregulated miRNAs, miR-125b-1-3p and miR-1183, were confirmed by qRT-PCR in 18 paired KS samples. The qRT-PCR results for 10 miRNAs were consistent with our microarray results. The miR-125b-1-3p and miR-16-5p had statistically significant associations with HHV-8 and HIV infections in KS. The results of miRNA profiling showed that KS appears to have unique expression patterns when compared with paired adjacent healthy tissues, suggesting that deregulation of miRNAs plays an important role in the progression of KS. These differentially expressed miRNAs may provide novel diagnostic and prognostic tools.




Similar content being viewed by others
References
Zeng Y. Principles of micro-RNA production and maturation. Oncogene. 2006;25(46):6156–62.
Engels BM, Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene. 2006;25(46):6163–9.
Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–5.
Farh KK-H, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, et al. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science. 2005;310(5755):1817–21.
Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific microRNAs. Dev Cell. 2003;5(2):351–8.
Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901–6.
Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9(3):219–30.
Hengge UR, Ruzicka T, Tyring SK, Stuschke M, Roggendorf M, Schwartz RA, et al. Update on kaposi’s sarcoma and other hhv8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect Dis. 2002;2(5):281–92.
Wang HW, Trotter MW, Lagos D, Bourboulia D, Henderson S, Makinen T, et al. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet. 2004;36(7):687–93.
Kaposi M. Idiopathic multiple pigmented sarcoma of the skin. Arch Dermatol Syphilol. 1872;4(6):265–73.
Kaloterakis A, Papasteriades C, Filiotou A, Economidou J, Hadjiyannis S, Stratigos J. Hla in familial and nonfamilial mediterranean Kaposi’s sarcoma in Greece. Tissue Antigens. 1995;45(2):117–9.
Dilnur P, Katano H, Wang ZH, Osakabe Y, Kudo M, Sata T, et al. Classic type of Kaposi’s sarcoma and human herpesvirus 8 infection in Xinjiang, China. Pathol Int. 2001;51(11):845–52.
Pu X-M, Wu W-D, Ju H-E. The detection of hhv-8 in the serum of Kaposi’s sarcoma before and after the therapy with interferon. J Clin Dermatol. 2004;33(2):87–8.
He F, Wang X, He B, Feng Z, Lu X, Zhang Y, et al. Human herpesvirus 8: serovprevalence and correlates in tumor patients from Xinjiang, China. J Med Virol. 2007;79(2):161–6.
Wang X, He B, Zhang Z, Liu T, Wang H, Li X, et al. Human herpesvirus-8 in northwestern China: epidemiology and characterization among blood donors. Virol J. 2010;7(1):1–7.
Wu XJ, Pu XM, Kang XJ, Halifu Y, An CX, Zhang DZ, Yakeya B, Mijit J. One hundred and five kaposi sarcoma patients: A clinical study in xinjiang, northwest of china. J Eur Acad Dermatol Venereol. 2014.
Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, et al. Identification of herpesvirus-like DNA sequences in aids-associated Kaposi’s sarcoma. Science. 1994;266(5192):1865–9.
Walter BA, Valera VA, Pinto PA, Merino MJ. Comprehensive microRNA profiling of prostate cancer. J Cancer. 2013;4(5):350–7.
Gu H, Li H, Zhang L, Luan H, Huang T, Wang L, et al. Diagnostic role of microRNA expression profile in the serum of pregnant women with fetuses with neural tube defects. J Neurochem. 2012;122(3):641–9.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative ct method. Nat Protoc. 2008;3(6):1101–8.
Jackson RJ, Standart N. How do microRNAs regulate gene expression? Sci Signal. 2007;2007(367):re1.
Zhang B, Pan X, Cobb GP, Anderson TA. MicroRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302(1):1–12.
Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–14.
Ene AMC, Borze I, Guled M, Costache M, Leen G, Sajin M, et al. MicroRNA expression profiles in Kaposi’s sarcoma. Pathol Oncol Res. 2014;20(1):153–9.
O’Hara AJ, Wang L, Dezube BJ, Harrington WJ, Damania B, Dittmer DP. Tumor suppressor microRNAs are underrepresented in primary effusion lymphoma and Kaposi sarcoma. Blood. 2009;113(23):5938–41.
Chen L, Yang Q, Kong WQ, Liu T, Liu M, Li X, et al. MicroRNA-181b targets camp responsive element binding protein 1 in gastric adenocarcinomas. IUBMB Life. 2012;64(7):628–35.
Nurul S, Yoke-Kqueen C, Sabariah AR, Shiran MS, Singh A, Learn-Han L. Differential microRNA expression and identification of putative miRNA targets and pathways in head and neck cancers. Int J Mol Med. 2011;28(3):327–36.
Ratert N, Meyer H-A, Jung M, Mollenkopf H-J, Wagner I, Miller K, et al. Reference miRNAs for miRNAome analysis of urothelial carcinomas. PLoS One. 2012;7(6):e39309.
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li M, et al. Mirna-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor epb41l3. Mol Cancer Res. 2011;9(7):824–33.
He J, Jing Y, Li W, Qian X, Xu Q, Li F-S, et al. Roles and mechanism of mir-199a and mir-125b in tumor angiogenesis. PLoS One. 2013;8(2):e56647.
Emmrich S, Henke K, Hegermann J, Ochs M, Reinhardt D, Klusmann J-H. MiRNAs can increase the efficiency of ex vivo platelet generation. Ann Hematol. 2012;91(11):1673–84.
Li X, Wang H-l, Peng X, Zhou H-l, Wang X. Mir-1297 mediates pten expression and contributes to cell progression in LSCC. Biochem Biophys Res Commun. 2012;427(9):254–60.
Roy D, Dittmer DP. Phosphatase and tensin homolog on chromosome 10 is phosphorylated in primary effusion lymphoma and Kaposi’s sarcoma. Am J Pathol. 2011;179(4):2108–19.
Parker LH, Schmidt M, Jin S-W, Gray AM, Beis D, Pham T, et al. The endothelial-cell-derived secreted factor egfl7 regulates vascular tube formation. Nature. 2004;428(6984):754–8.
Meister J, Schmidt MHH. Mir-126 and mir-126*: new players in cancer. Sci World J. 2010;10(9):2090–100.
Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, et al. The Kaposi’s sarcoma-associated herpes virus g protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1 alpha. Cancer Res. 2000;60(17):4873–80.
Cai X, Lu S, Zhang Z, Gonzalez CM, Damania B, Cullen BR. Kaposi’s sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc Natl Acad Sci U S A. 2005;102(15):5570–5.
Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, Chi JT, et al. A viral microRNA functions as an orthologue of cellular mir-155. Nature. 2007;450(7172):1096–9.
Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, et al. Kaposi’s sarcoma-associated herpesvirus encodes an ortholog of mir-155. J Virol. 2007;81(23):12836–45.
Acknowledgments
This work was supported by a grant from the Natural Science Foundation of China (Grant No. 81260311) and Natural Science Foundation of Xinjiang Uygur Autonomous Region (Grant No. 2014211A059). We are most grateful to all the families who have so willingly participated in this study and made this study possible. We also acknowledge the support of the Clinical Dermatology Institute of Xinjiang Uygur Autonomous Region and National Clinical key specialty construction projects.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiu-Juan Wu and Xiong-Ming Pu contributed equally to this work and should be considered joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 14 kb)
Supplementary Table 2
(DOCX 18 kb)
Supplementary Table 3
(DOCX 18 kb)
Supplementary Figure 1
(DOCX 412 kb)
Supplementary Figure 2
(DOCX 357 kb)
Supplementary Figure 3
(DOCX 238 kb)
Rights and permissions
About this article
Cite this article
Wu, XJ., Pu, XM., Zhao, ZF. et al. The expression profiles of microRNAs in Kaposi’s sarcoma. Tumor Biol. 36, 437–446 (2015). https://doi.org/10.1007/s13277-014-2626-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2626-1