Abstract
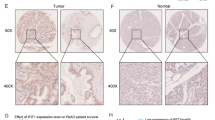
ARMC8 proteins are novel armadillo repeat containing proteins, which are well conserved in eukaryotes and are involved in a variety of processes such as cell migration, proliferation, tissue maintenance, signal transduction, and tumorigenesis. Armadillo repeat proteins include well-known proteins such as β-catenin and p120ctn. Our current knowledge of ARMC8, especially its role in cancer, is limited. In this study, we quantified ARMC8 expression in 112 non-small cell lung cancer (NSCLC) tissues and adjacent non-cancerous tissues, and seven lung cancer cell lines using immunohistochemistry staining and Western blotting. ARMC8 level was significantly higher in NSCLC tissues than in the adjacent normal tissues (67.9 % versus 5.4 %, p < 0.05) and was significantly associated with TNM stage (p = 0.022), lymph node metastasis (p = 0.001), and poor prognosis (p < 0.001) in NSCLC patients. Cox regression analysis demonstrated that ARMC8 was an independent prognostic factor for NSCLC. Consistent with this, ARMC8α downregulation by siRNA knockdown inhibited growth, colony formation, and invasion in A549 lung cancer cells, while ARMC8α overexpression promoted growth, colony formation, and invasion in H1299 lung cancer cells. In addition, ARMC8α knockdown downregulated canonical Wnt-signaling pathway activity and cyclin D1 and matrix metalloproteinase (MMP)-7 expression. Consistent with this, ARMC8α overexpression upregulated canonical Wnt-signaling pathway activity and cyclin D1 and MMP-7 expression. These results indicate that ARMC8α upregulates cyclin D1 and MMP7 expression by activating the canonical Wnt-signaling pathway and thereby promoting lung cancer cell proliferation and invasion. Therefore, ARMC8 might serve as a novel therapeutic target in NSCLC.






Similar content being viewed by others
References
Riggleman B, Wieschaus E, Schedl P. Molecular analysis of the armadillo locus: uniformly distributed transcripts and a protein with novel internal repeats are associated with a Drosophila segment polarity gene. Genes Dev. 1989;3:96–113.
Coates JC. Armadillo repeat proteins: beyond the animal kingdom. Trends Cell Biol. 2003;13:463–71.
Gorlich D, Prehn S, Laskey RA, et al. Isolation of a protein that is essential for the first step of nuclear protein import. Cell. 1994;79:767–78.
McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–61.
Franke WW, Goldschmidt MD, Zimbelmann R, et al. Molecular cloning and amino acid sequence of human plakoglobin, the common junctional plaque protein. Proc Natl Acad Sci U S A. 1989;86:4027–31.
Kinzler KW, Nilbert MC, Vogelstein B, et al. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991;251:1366–70.
Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600.
Reynolds AB, Herbert L, Cleveland JL, et al. p120, a novel substrate of protein tyrosine kinase receptors and of p60v-src, is related to cadherin-binding factors β-catenin, plakoglobin and armadillo. Oncogene. 1992;7:2439–45.
Hatzfeld M, Kristjanssen GI, Plessmann U, et al. Band 6 protein, a major constituent of desmosomes from stratified epithelia, is a novel member of the armadillo multigene family. J Cell Sci. 1994;107:2259–70.
Hatzfeld M. The armadillo family of structural proteins. Int Rev Cytol. 1999;186:179–224.
Kobayashi N, Yang J, Ueda A, et al. RanBPM, Muskelin, p48EMLP, p44CTLH, and the armadillo-repeat proteins ARMC8α and ARMC8β are components of the CTLH complex. Gene. 2007;396:236–47.
Tomaru K, Ueda A, Suzuki T, et al. Armadillo repeat containing 8alpha binds to HRS and promotes HRS interaction with ubiquitinated proteins.Open. Biochem J. 2010;4:1–8.
Suzuki T, Ueda A, Kobayashi N, et al. Proteasome-dependent degradation of alpha-catenin is regulated by interaction with ARMc8alpha. Biochem J. 2008;411(3):581–91.
Liu Y, Zheng J, Fang W, et al. Isolation and characterization of human prostate cancer cell subclones with different metastatic potential. Zhonghua Bing Li Xue Za Zhi. 1999;28:361–4.
Zhu W, Zheng J, Fang W. Isolation and characterization of human lung cancer cell subline with different metastatic potential. Zhonghua Bing Li Xue Za Zhi. 1995;24:136–8.
Hwang SG, Yu SS, Ryu JH, et al. Regulation of beta-catenin signaling and maintenance of chondrocyte differentiation by ubiquitin-independent proteasomal degradation of alpha-catenin. J Biol Chem. 2005;280(13):12758–65.
Pestell RG. New roles of cyclin D1. Am J Pathol. 2013;183(1):3–9.
Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23(1-2):101–17.
Karim R, Tse G, Putti T, et al. The significance of the Wnt pathway in the pathology of human cancers. Pathology. 2004;36(2):120–8.
Kolligs FT, Bommer G, Göke B. Wnt/beta-catenin/tcf signaling: a critical pathway in gastrointestinal tumorigenesis. Digestion. 2002;66(3):131–44.
Vasioukhin V, Fuchs E. Actin dynamics and cell–cell adhesion in epithelia. Curr Opin Cell Biol. 2001;13:76–84.
Kobielak A, Fuchs E. α-Catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5:614–25.
Mege RM, Gavard J, Lambert M. Regulation of cell–cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541–8.
Acknowledgments
The authors thank Prof. Ishigatsubo Y, Department of Internal Medicine and Clinical Immunology, Yokohama City University Graduate School of Medicine, for kindly providing ARMC8 pcDNA. This study was supported by the National Natural Science Foundation of China (No. 81272606 to Enhua Wang, No. 81301837 to Juanhan Yu and No.81101779 to Di Zhang).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, C., Jiang, G., Fan, C. et al. ARMC8α promotes proliferation and invasion of non-small cell lung cancer cells by activating the canonical Wnt signaling pathway. Tumor Biol. 35, 8903–8911 (2014). https://doi.org/10.1007/s13277-014-2162-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2162-z