Abstract
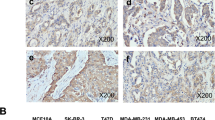
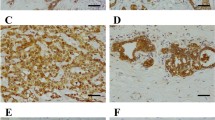
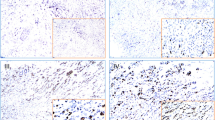
Breast cancer is the most common cancer in women worldwide. Studies have shown that the high temperature requirement factor A3 (HtrA3) is involved in important physiological processes including maintenance of mitochondrial homeostasis, cell death, and cell signaling. HtrA3 is reported to be downregulated in several cancers and has been correlated with advancing cancer stage. We performed a retrospective study using our breast cancer tissue bank to investigate whether the expression of HtrA3 correlated with lymphatic metastasis in breast cancer and whether the expression of HtrA3 was correlated with estrogen receptor (ER) and progesterone receptor (PR) expression in breast cancer. Breast cancer tissues from 156 invasive ductal breast cancer patients with or without lymphatic metastasis were collected and the levels of HtrA3 were measured by immunohistochemistry and western blotting. The expression of HtrA3 was lower in breast cancer. In particular, HtrA3 expression in breast cancer with lymphatic metastasis was lower than that in breast cancer without lymphatic metastasis. In breast cancers with no lymphatic metastasis, the expression of HtrA3 was lower in patients with ER- and PR-positive tumors, but when breast cancers with lymphatic metastasis were analyzed, there was no difference in HtrA3 expression between ER- and PR-positive or ER- and PR-negative tumors. These data suggest that the expression of HtrA3 was negatively correlated with lymphatic metastasis in breast cancer but not correlated with ER and PR positivity or negativity. A better understanding of the mechanism of HtrA3 may provide the basis for future development of a novel therapeutic target in breast cancer.




Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22.
Donegan WL. Tumor-related prognostic factors for breast cancer. CA Cancer J Clin. 1997;47:28–51.
Fisher ER, Kotwal N, Hermann C, et al. Types of tumor lymphoid response and sinus histiocytosis. Relationship to five-year, disease-free survival in patients with breast cancer. Arch Pathol Lab Med. 1983;107:222–7.
Clausen T, Southan C, Ehrmann M. The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell. 2002;10:443–55.
Zurawa-Janicka D, Skorko-Glonek J, Lipinska B. HtrA proteins as targets in therapy of cancer and other diseases. Expert Opin Ther Targets. 2010;14:665–79.
Clausen T, Kaiser M, Huber R, et al. HTRA proteases: regulated proteolysis in protein quality control. Nat Rev Mol Cell Biol. 2011;12:152–62.
Chien J, Campioni M, Shridhar V, Baldi A. HtrA serine proteases as potential therapeutic targets in cancer. Curr Cancer Drug Targets. 2009;9:451–68.
Nie G, Li Y, Hale K, et al. Serine peptidase HTRA3 is closely associated with human placental development and is elevated in pregnancy serum. Biol Reprod. 2006;74:366–74.
Nie GY, Hampton A, Li Y, et al. Identification and cloning of two isoforms of human high-temperature requirement factor A3 (HtrA3), characterization of its genomic structure and comparison of its tissue distribution with HtrA1 and HtrA2. Biochem J. 2003;371:39–48.
Nie GY, Li Y, Minoura H, et al. A novel serine protease of the mammalian HtrA family is up-regulated in mouse uterus coinciding with placentation. Mol Hum Reprod. 2003;9:279–90.
Beleford D, Liu Z, Rattan R, et al. Methylation induced gene silencing of HtrA3 in smoking-related lung cancer. Clin Cancer Res. 2010;16:398–409.
Beleford D, Rattan R, Chien J, et al. High temperature requirement A3 (HtrA3) promotes etoposide- and cisplatin-induced cytotoxicity in lung cancer cell lines. J Biol Chem. 2010;285:12011–27.
Bowden MA, Di Nezza-Cossens LA, Jobling T, et al. Serine proteases HTRA1 and HTRA3 are down-regulated with increasing grades of human endometrial cancer. Gynecol Oncol. 2006;103:253–60.
Narkiewicz J, Klasa-Mazurkiewicz D, Zurawa-Janicka D, et al. Changes in mRNA and protein levels of human HtrA1, HtrA2 and HtrA3 in ovarian cancer. Clin Biochem. 2008;41:561–9.
Narkiewicz J, Lapinska-Szumczyk S, Zurawa-Janicka D, et al. Expression of human HtrA1, HtrA2, HtrA3 and TGF-beta1 genes in primary endometrial cancer. Oncol Rep. 2009;21:1529–37.
Singh HL, Fuller PJ, Harrison C, et al. HtrA3 is downregulated in cancer cell lines and significantly reduced in primary serous and granulosa cell ovarian tumors. J Cancer. 2013;4(2):152–64.
de Boer M, van Deurzen CH, van Dijck JA, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009;361:653–63.
Neuhausen SL, Brummel S, Ding YC, et al. Genetic variation in IGF2 and HTRA1 and breast cancer risk among BRCA1 and BRCA2 carriers. Cancer Epidemiol Biomarkers Prev. 2011;20:1690–702.
Chien J, Staub J, Hu SI, et al. A candidate tumor suppressor HtrA1 is downregulated in ovarian cancer. Oncogene. 2004;23:1636–44.
Zumbrunn J, Trueb B. Primary structure of a putative serine protease specific for IGF-binding proteins. FEBS Lett. 1996;398:187–92.
Hammond ME, Hayes DF, Wolff AC, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–7.
Dynon K, Heng S, Puryer M, et al. HtrA3 as an early marker for preeclampsia: specific monoclonal antibodies and sensitive high-throughput assays for serum screening. PLoS One. 2012;7:e45956.
Soslow RA, Dannenberg AJ, Rush D, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–45.
Society AC. Cancer facts and figures. Atlanta: Society AC; 2010.
Baldi A, De Luca A, Morini M, et al. The HtrA1 serine protease is down-regulated during human melanoma progression and represses growth of metastatic melanoma cells. Oncogene. 2002;21:6684–8.
Tocharus J, Tsuchiya A, Kajikawa M, et al. Developmentally regulated expression of mouse HtrA3 and its role as an inhibitor of TGF-beta signaling. Dev Growth Differ. 2004;46:257–74.
Dumont N, Arteaga CL. Targeting the TGF beta signaling network in human neoplasia. Cancer Cell. 2003;3:531–6.
Singh H, Endo Y, Nie G. Decidual HtrA3 negatively regulates trophoblast invasion during human placentation. Hum Reprod. 2011;26:748–57.
Singh H, Makino SI, Endo Y, et al. Inhibition of HTRA3 stimulates trophoblast invasion during human placental development. Placenta. 2010;31:1085–92.
Amir E, Seruga B, Serrano R, et al. Targeting DNA repair in breast cancer: a clinical and translational update. Cancer Treat Rev. 2010;36:557–65.
Acknowledgments
This study was supported by the Chinese National Nature Sciences Foundation (grant number 81100437 to M Zhao) and Australian National and Health and Medical Research Council (Senior Research Fellowship GNT1041835 to G Nie). Authors thank all the women who donated tissues for this study. The authors also thank Dr. Joanna James from The University of Auckland for reviewing this manuscript.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Y Yin and M Wu equally contributed to this work.
Rights and permissions
About this article
Cite this article
Yin, Y., Wu, M., Nie, G. et al. HtrA3 is negatively correlated with lymph node metastasis in invasive ductal breast cancer. Tumor Biol. 34, 3611–3617 (2013). https://doi.org/10.1007/s13277-013-0942-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0942-5