Abstract
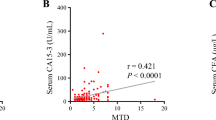
Neoadjuvant chemotherapy in breast cancer patients aims at preoperative reduction of tumor volume for better resection results and prognosis. As not all patients respond to neoadjuvant therapy, predictive biomarkers are needed for more efficient individual management. In prospectively collected sera of 51 consecutive locally confined breast cancer (LBC) patients receiving preoperative, neoadjuvant chemotherapy, value level kinetics of soluble high mobility group box 1 (HMGB1), soluble receptor for advanced glycation end products (sRAGE) as well as the established breast cancer biomarkers CA 15–3 and carcinoembryonic antigen (CEA) were investigated and correlated with therapy response objectified by pathological staging at surgery. In addition, biomarkers were measured in sera of 30 healthy controls (HC), 13 patients with benign breast diseases, and 28 metastatic breast cancer (MBC) patients. Pretherapeutic levels of soluble HMGB1 were decreased in MBC, while sRAGE was already decreased in LBC. In contrast, CA 15–3 and CEA were strongly elevated in MBC, but not in LBC. Combination of sRAGE and CA 15–3 enabled best discrimination of LBC from HC (AUC 78.2 %; sens 58 % at 95 % spec), while CA15-3 and CEA discriminated best between MBC and all controls (AUC 90.9 %; sens 70 % at 95 % spec). In LBC patients undergoing neoadjuvant chemotherapy, nine patients achieved complete remission (CR), 29 achieved partial remission (PR), while 13 had no change of disease (NC). NC patients tended to have higher HMGB1 and lower sRAGE levels before therapy onset (p = 0.056 and p = 0.054), while CA 15–3 and CEA did not predict therapeutic outcome. Furthermore, kinetics of HMGB1 during therapy correlated with efficacy of the treatment (p = 0.053). Markers of immunogenic cell death are valuable for the diagnosis of MBC and early estimation of response to neoadjuvant therapy in LBC patients.



Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Ozols RF, Herbst RS, Colson YL, et al. American Society of Clinical Oncology. Clinical cancer advances 2006: major research advances in cancer treatment, prevention, and screening—a report from the American Society of Clinical Oncology. J Clin Oncol. 2007;25:146–62.
Bundret NJ. Prognostic and predictive factors in breast cancer. Cancer Trial Rev. 2001;27:137–42.
Von Minckwitz G. Neoadjuvant chemotherapy in breast cancer—insights from the German experience. Breast Cancer 2012; published online.
EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Early Breast Cancer Trialists Collaborative Group. Lancet. 2005;365:1687–717.
Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102.
Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85.
Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant M, Harris JR, Karn T, Liedtke C, Mauri D, Rouzier R, Ruckhaeberle E, Semiglazov V, Symmans WF, Tutt A, Pusztai L. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–16.
Beachy SH, Repasky EA. Using extracellular biomarkers for monitoring efficacy of therapeutics in cancer patients: an update. Cancer Immunol Immunother. 2008;57:759–75.
Sturgeon CM, Duffy MJ, Stenman UH, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54:e11–79.
Holdenrieder S, Stieber P. Apoptotic markers in cancer. Clin Biochem. 2004;37:605–17.
Holdenrieder S, Stieber P. Clinical use of circulating nucleosomes. Crit Rev Lab Med Sci. 2009;46:1–24.
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88.
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73.
Holdenrieder S, Stieber P, von Pawel J, et al. Circulating nucleosomes predict the response to chemotherapy in patients with advanced non small cell lung cancer. Clin Cancer Res. 2004;10:5981–7.
Holdenrieder S, Stieber P, von Pawel J, et al. Early and specific prediction of the therapeutic efficacy in lung cancer by nucleosomal DNA and cytokeratin 19 fragments. Ann N Y Acad Sci. 2006;1075:244–75.
Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42.
Bianchi ME. HMGB1 loves company. J Leukocyte Biology. 2009;86:573–76.
Urbonaviciute V, Fürnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Herrmann M, Voll RE. Induction of inflammatory and immune responses by HMGB1–nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–18.
Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Molecular characteristics of immunogenic cancer cell death. Cell Death and Differentiation. 2008;15:3–12.
Park JS. G-RF, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, et al.: High mobility group box 1 protein interacts with multiple toll-like receptors. Am J Physiol Cell Physiol. 2006;290:917–24.
Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, Kroemer G. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14:364–75.
Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9.
Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68:4026–30.
Chung HW, Lee SG, Kim H, Hong DJ, Chung JB, Stroncek D, Lim JB. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J Transl Med. 2009;7:38.
Naumnik W, Nilklińska W, Ossolińska M, Chyczewska E. Serum levels of HMGB1, survivin, and VEGF in patients with advanced non-small cell lung cancer during chemotherapy. Folia Histochem Cytobiol. 2009;47:703–9.
Tang DL, Kang R, Zeh HJ, Lotze MT. High-mobility group box 1 and cancer. Biochimica Et Biophysica Acta-Gene Regulatory Mechanisms. 2010;1799:131–40.
Bierhaus A, Nawroth PP. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologica. 2009;52:2251–63.
Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT. RAGE (receptor for advanced glycation endproducts), RAGE ligands, and their role in cancer and inflammation. Journal of Translational Medicine. 2010;7:17.
Geroldi D, Falcone C, Emanuele E. Soluble receptor for advanced glycation end products: from disease marker to potential therapeutic target. Curr Med Chem. 2006;13:1971–8.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16.
Lehner J, Wittwer C, Fersching D, Siegele B, Holdenrieder S, Stoetzer OJ. Methodical and preanalytical evaluation of an HMGB1 immunoassay. Anticancer Res. 2012;32:2059–62.
Wittwer C, Lehner J, Fersching D, Siegele B, Stoetzer OJ, Holdenrieder S. Methodical and preanalytical evaluation of a RAGE immunoassay. Anticancer Res. 2012;32:2075–78.
Holdenrieder S, Nagel D, Stieber P. Estimation of prognosis by circulating biomarkers in patients with non-small cell lung cancer. Cancer Biomarkers. 2010;6:179–90.
Barak V, Holdenrieder S, Nisman B, Stieber P. Relevance of circulating biomarkers for the therapy monitoring and follow-up investigations in patients with non-small cell lung cancer. Cancer Biomarkers. 2010;6:191–6.
Kumar S, Guleria R, Singh V, Bharti AC, Mohan A, Das BC. Plasma nucleosome levels might predict response to therapy in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2010;11:36–44.
Holdenrieder S. v Pawel J, Dankelmann E, et al. Nucleosomes, ProGRP, NSE, CYFRA 21–1 and CEA in the therapy monitoring of small-cell lung cancer during first-line chemotherapy. Clin Cancer Res. 2008;14:7813–21.
Kremer A, Wilkowski R, Holdenrieder S, et al. Nucleosomes in pancreatic cancer patients during radiochemotherapy. Tumour Biol. 2005;26:44–9.
Kremer A, Holdenrieder S, Stieber P, et al. Nucleosomes in colorectal cancer patients during radiochemotherapy. Tumour Biol. 2006;27:235–42.
Stötzer OJ, Fersching DIM, Salat C, Siegele B, Nagel D, Holdenrieder S. Prediction of response to neoadjuvant chemotherapy in breast cancer patients by circulating circulating nucleosomes, DNAse activity, M30 and survivin. Cancer Lett, submitted
Jiao L, Weinstein SJ, Albanes D, Taylor PR, Graubard BI, Virtamo J, Stolzenberg-Solomon RZ. Evidence that serum levels of the soluble receptor for advanced glycation end products are inversely associated with pancreatic cancer risk: a prospective study. Cancer Res. 2011;71:3582–9.
Krechler T, Jáchymová M, Mestek O, Zák A, Zima T, Kalousová M. Soluble receptor for advanced glycation end-products (sRAGE) and polymorphisms of RAGE and glyoxalase I genes in patients with pancreas cancer. Clin Biochem. 2010;43:882–6.
Jiao L, Taylor PR, Weinstein SJ, Graubard BI, Virtamo J, Albanes D, Stolzenberg-Solomon RZ. Advanced glycation end products, soluble receptor for advanced glycation end products, and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1430–8.
Jing R, Cui M, Wang J, Wang H. Receptor for advanced glycation end products (RAGE) soluble form (sRAGE): a new biomarker for lung cancer. Neoplasma. 2010;57:55–61.
Tesarová P, Kalousová M, Jáchymová M, Mestek O, Petruzelka L, Zima T. Receptor for advanced glycation end products (RAGE)—soluble form (sRAGE) and gene polymorphisms in patients with breast cancer. Cancer Invest. 2007;25:720–5.
Shang GH, Jia CQ, Tian H, Xiao W, Li Y, Wang AH, Dong L, Lin DJ. Serum high mobility group box protein 1 as a clinical marker for non-small cell lung cancer. Respir Med. 2009;103:1949–53.
Cheng BQ, Jia CQ, Liu CT, Lu XF, Zhong N, Zhang ZL, Fan W, Li YQ. Serum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Dig Liver Dis. 2008;40:446–52.
Sheng X, Du X, Zhang X, Li D, Lu C, Li Q, Ma Z, Song Q, Wang C. Clinical value of serum HMGB1 levels in early detection of recurrent squamous cell carcinoma of uterine cervix: comparison with serum SCCA, CYFRA21-1, and CEA levels. Croat Med J. 2009;50:455–64.
Urbonaviciute V, Fürnrohr BG, Weber C, Haslbeck M, Wilhelm S, Herrmann M, Voll RE. Factors masking HMGB1 in human serum and plasma. J Leukoc Biol. 2007;81:67–74.
Molina R, Barak V, van Dalen A, Duffy MJ, Einarsson R, Gion M, Goike H, Lamerz R, Nap M, Sölétormos G, Stieber P. Tumor markers in breast cancer—European Group on Tumor Markers recommendations. Tumour Biol. 2005;26:281–93.
Laessig D, Nagel D, Heinemann V, Untch M, Kahlert S, Bauerfeind I, Stieber P. Importance of CEA and CA 15–3 during disease progression in metastatic breast cancer patients. Anticancer Res. 2007;27:1963–8.
Molina R, Gion M, Gressner A, Troalen F, Auge JM, Holdenrieder S, Zancan M, Wycislo M, Stieber P. Alternative antibody for the detection of CA15-3 antigen: a European multicenter study for the evaluation of the analytical and clinical performance of the Access BR Monitor assay on the UniCel Dxl 800 Immunoassay System. Clin Chem Lab Med. 2008;46:612–22.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(PPT 503 kb)
Rights and permissions
About this article
Cite this article
Stoetzer, O.J., Fersching, D.M.I., Salat, C. et al. Circulating immunogenic cell death biomarkers HMGB1 and RAGE in breast cancer patients during neoadjuvant chemotherapy. Tumor Biol. 34, 81–90 (2013). https://doi.org/10.1007/s13277-012-0513-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0513-1