Abstract
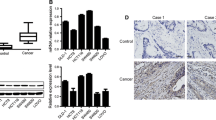
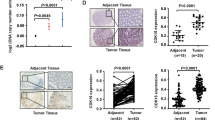
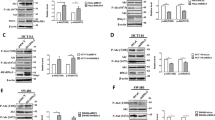
P21-activated protein kinase1 (PAK1), a main downstream effector of small Rho GTPases, Rac1, and Cdc42, plays an important role in the regulation of cell morphogenesis, motility, mitosis, and angiogenesis. Despite its importance, the molecular mechanisms of PAK1 that contributed to colorectal carcinogenesis remain unclear. Our immunohistochemistry showed that PAK1 expression was increased with colorectal cancer (CRC) progression through the adenoma to carcinoma sequence. Furthermore, our results suggested a relationship between PAK1 nuclear localization and the Dukes staging. In the present study, we showed that PAK1 knockdown decreased proliferation and delayed the G1/S cell-cycle transition, and increased apoptosis in vivo and in vitro. In addition, PAK1 knock-down downregulated c-Jun amino terminal kinases (JNK) activity and the levels of cyclinD1, CDK4/6. Inhibition of the JNK activity by chemical inhibitor (SP600125) significantly reduced the effects of PAK1 on CRC proliferation via accumulation of phosphatase and tensin homolog deleted on chromosome 10 (PTEN). In conclusion, our results demonstrate that knockdown of PAK1 could enhance the chemosensitivity of CRCs to 5-fluorouracil through G1 arrest. The mechanism by which PAK1 induced cancer growth might involve activation of JNK as well as downregulation of PTEN. Targeting PAK1 may represent a novel treatment strategy for developing novel chemotherapeutic agents.




Similar content being viewed by others
References
Jones Jr DV, Winn RJ, Brown BW, Levy LB, Pugh RP, Wade 3rd JL, Gross HM, Pendergrass KB, Levin B, Abbruzzese JL. Randomized phase III study of 5-fluorouracil plus high dose folinic acid versus 5-fluorouracil plus folinic acid plus methyl-lomustine for patients with advanced colorectal cancer. Cancer. 1995;76:1709–14.
Marsoni S. Fluorouracil and folinic acid in colon cancer. IMPACT Investigators. Lancet. 1995;345:1582–3.
Adam L, Vadlamudi R, Mandal M, Chernoff J, Kumar R. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J Biol Chem. 2000;275:12041–50.
Liu F, Li X, Wang C, Cai X, Du Z, Xu H, Li F. Downregulation of p21-activated kinase-1 inhibits the growth of gastric cancer cells involving cyclin B1. Int J Cancer. 2009;125:2511–9.
O'Sullivan GC, Tangney M, Casey G, Ambrose M, Houston A, Barry OP. Modulation of p21-activated kinase 1 alters the behavior of renal cell carcinoma. Int J Cancer. 2007;121:1930–40.
Li F, Adam L, Vadlamudi RK, Zhou H, Sen S, Chernoff J, Mandal M, Kumar R. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep. 2002;3:767–73.
Maroto B, Ye MB, von Lohneysen K, Schnelzer A, Knaus UG. P21-activated kinase is required for mitotic progression and regulates Plk1. Oncogene. 2008;27:4900–8.
Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–49.
Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, Kumar R. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem. 2004;279:1422–8.
Wang RA, Zhang H, Balasenthil S, Medina D, Kumar R. PAK1 hyperactivation is sufficient for mammary gland tumor formation. Oncogene. 2006;25:2931–6.
Aoki H, Yokoyama T, Fujiwara K, Tari AM, Sawaya R, Suki D, Hess KR, Aldape KD, Kondo S, Kumar R, Kondo Y. Phosphorylated Pak1 level in the cytoplasm correlates with shorter survival time in patients with glioblastoma. Clin Cancer Res. 2007;13:6603–9.
Schraml P, Schwerdtfeger G, Burkhalter F, Raggi A, Schmidt D, Ruffalo T, King W, Wilber K, Mihatsch MJ, Moch H. Combined array comparative genomic hybridization and tissue microarray analysis suggest PAK1 at 11q13.5–q14 as a critical oncogene target in ovarian carcinoma. Am J Pathol. 2003;163:985–92.
Gong W, An Z, Wang Y, Pan X, Fang W, Jiang B, Zhang H. P21-activated kinase 5 is overexpressed during colorectal cancer progression and regulates colorectal carcinoma cell adhesion and migration. Int J Cancer. 2009;125:548–55.
Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404.
Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6:322–7.
Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–65.
Mariadason JM, Arango D, Shi Q, Wilson AJ, Corner GA, Nicholas C, Aranes MJ, Lesser M, Schwartz EL, Augenlicht LH. Gene expression profiling-based prediction of response of colon carcinoma cells to 5-fluorouracil and camptothecin. Cancer Res. 2003;63:8791–812.
Dowdy SF, Hinds PW, Louie K, Reed SI, Arnold A, Weinberg RA. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499–511.
Ezhevsky SA, Ho A, Becker-Hapak M, Davis PK, Dowdy SF. Differential regulation of retinoblastoma tumor suppressor protein by G(1) cyclin-dependent kinase complexes in vivo. Mol Cell Biol. 2001;21:4773–84.
Ezhevsky SA, Nagahara H, Vocero-Akbani AM, Gius DR, Wei MC, Dowdy SF. Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc Natl Acad Sci USA. 1997;94:10699–704.
Harbour JW, Luo RX. Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–69.
Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin–cdk complexes. Mol Cell Biol. 1998;18:753–61.
Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–32.
Pedram A, Razandi M, Levin ER. Extracellular signal-regulated protein kinase/Jun kinase cross-talk underlies vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem. 1998;273:26722–8.
Zhang JY, Tao S, Kimmel R, Khavari PA. CDK4 regulation by TNFR1 and JNK is required for NF-kappaB-mediated epidermal growth control. J Cell Biol. 2005;168:561–6.
Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–400.
Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52.
Kuntzen C, Sonuc N, De Toni EN, Opelz C, Mucha SR, Gerbes AL, Eichhorst ST. Inhibition of c-Jun-N-terminal-kinase sensitizes tumor cells to CD95-induced apoptosis and induces G2/M cell cycle arrest. Cancer Res. 2005;65:6780–8.
Potapova O, Gorospe M, Bost F, Dean NM, Gaarde WA, Mercola D, Holbrook NJ. c-Jun N-terminal kinase is essential for growth of human T98G glioblastoma cells. J Biol Chem. 2000;275:24767–75.
Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15:713–25.
Poitras L, Jean S, Islam N, Moss T. PAK interacts with NCK and MLK2 to regulate the activation of jun N-terminal kinase. FEBS Lett. 2003;543:129–35.
Zhou L, Yan C, Gieling RG, Kida Y, Garner W, Li W, Han YP. Tumor necrosis factor-alpha induced expression of matrix metalloproteinase-9 through p21-activated kinase-1. BMC Immunol. 2009;10:15.
Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51–63.
Denning MF, Wang Y, Tibudan S, Alkan S, Nickoloff BJ, Qin JZ. Caspase activation and disruption of mitochondrial membrane potential during UV radiation-induced apoptosis of human keratinocytes requires activation of protein kinase C. Cell Death Differ. 2002;9:40–52.
Zang M, Hayne C, Luo Z. Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1. J Biol Chem. 2002;277:4395–405.
Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J Biol Chem. 2005;280:24698–705.
Schurmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, Bokoch GM. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol. 2000;20:453–61.
Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8.
Tokunaga E, Oda S, Fukushima M, Maehara Y, Sugimachi K. Differential growth inhibition by 5-fluorouracil in human colorectal carcinoma cell lines. Eur J Cancer. 2000;36:1998–2006.
Violette S, Poulain L, Dussaulx E, Pepin D, Faussat AM, Chambaz J, Lacorte JM, Staedel C, Lesuffleur T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int J Cancer. 2002;98:498–504.
Acknowledgments
Grant support: The Nature Science Foundation of China grant 30830048 and 973 Program of the Ministry of Science and Technology of China (2010CB912203) to Hongquan Zhang. We thank Dr. Feici Diao for critical reading of the manuscript, Dr. Hua Tian for providing the pcDNA3.1(+)-PTEN and vector plasmids, and Dr. Yan Sun for helpful advice with the siRNA, cell cycle, and apoptosis experiments.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Haitao Qing and Wei Gong contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Nuclear localization of PAK1. (a) and (b) are representatives which have strong PAK1 immunoactivity in the nucleus as well as in the cytoplasm. a, b: ×400 (JPEG 26 kb)
Rights and permissions
About this article
Cite this article
Qing, H., Gong, W., Che, Y. et al. PAK1-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Tumor Biol. 33, 985–994 (2012). https://doi.org/10.1007/s13277-012-0327-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0327-1