Abstract
Backgrounds Alzheimer’s disease (AD) and fronto-temporal dementia (FTD) are the two most common neurodegenerative diseases leading to early onset dementia (<65 years). Mutations in the amyloid precursor protein, presenilin, and presenilin 2 genes are involved in some cases of familial early-onset AD (EOAD), while the microtubule-associated protein tau (MAPT) and pro-granulin (GRN) mutations have been mainly identifed in FTD patients. Clinically, FTD was often misdiag-nosed and confused with AD or psychiatric disorders, which could be a challenge in disease diagnosis.
Methods: We performed mutation analysis of GRN in 89 Korean patients with clinically diagnosed EOAD. In silico predictions were also performed for the variants to estimate their role in different disorders.
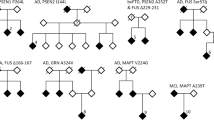
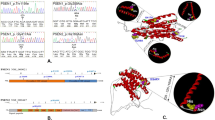
Results: No pathological mutations in MAPT was identified, but we identified two novel genetic variations in the GRN gene: p.Leu585Phe (c.1767G>T) and c.IVS8+23_+26delTGGG, which occurred independently in two EOAD patients (frequenct of 2/89, 2.2%). Using a combination of clinical and association studies, in silico prediction, and 3-D modeling software, we suggest that both mutations are probably pathogenic and involved in FTD.
Conclusion: Our data suggest that it would be important to re-examine EOAD patients who had been diagnosed when the FTD spectrum was not well described and the causative FTD genes had not yet been identifed. In addition, we propose initially analyzing genes associated with the frst form of suspected dementia and, if the results are negative, studying genes implicated in the other form of dementia.
Similar content being viewed by others
References
Giau, V. V., Bagyinszky, E., An, S. S. & Kim, S. Y. Clinical genetic strategies for early onset neurodegenerative diseases. Mol Cell Toxicol 14, 123 (2018).
Goldman, J. S. et al. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology 65, 1817–1819(2005).
Goldman, J. S., Adamson, J., Karydas, A., Miller, B. L. & Hutton, M. New genes, new dilemmas: FTLD genetics and its implications for families. Am J Alzheimers Dis Other Demen 22, 507–515. (2008).
Seelaar, H., Rohrer, J. D., Pijnenburg, Y. A., Fox, N. C. & van Swieten, J. C. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Phychiatry 82, 476–486 (2011).
Kim, E. J. et al. Clinical and genetic analysis of MAPT, GRN, and C9orf72 genes in Korean patients with frontotemporal dementia. Neurobiol Aging 35, 1213.e13–17 (2014).
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944(1984).
Bora, E., Walterfang, M. & Velakoulis, D. Theory of mind in behavioural-variant frontotemporal dementia and Alzheimer’s disease: a meta-analysis. J Neurol Neurosurg Psychiatr 86, 714–719 (2015).
Mega, M. S. et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology 46, 130–135(1996).
Ji, Y. et al. Apolipoprotein E ε4 frequency is increased among Chinese patients with frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord 36, 163–170(2013).
Mendez, M. F. & McMurtray, A. Frontotemporal dementia-like phenotypes associated with presenilin-1 mutations. Am J Alzheimers Dis Other Demen 21, 281–286 (2006).
Rabinovici, G. D. & Miller, B. L. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs 24, 375–398 (2010).
Spillantini, M. G. & Goedert, M. Tau pathology and neurodegeneration. Lancet Neurol 12, 609–622(2013).
Giau, V. V., Senanarong, V., Bagyinszky, E., An, S. S. A. & Kim, S. Y. Analysis of 50 Neurodegenerative Genes in Clinically Diagnosed Early-Onset Alzheimer’s Disease. Int J Mol Sci 26, E1514(2019). doi: 10.3390/ ijms20061514.
Gallo, M. et al. The novel PSEN1 M84V mutation associated to frontal dysexecutive syndrome, spastic paraparesis, and cerebellar atrophy in a dominant Alzheimer’s disease family. Neurobiol Aging 56, 213.e7–213.e12 (2017).
Lombardi, G. et al. Low florbetapir PET uptake and normal A1-42 cerebrospinal fuid in an APP Ala713Thr mutation carrier. J Alzheimers Dis 57, 697–703 (2017).
Giau, V. V., Bagyinszky, E., An, S. S. & Kim, S. Y. Gene panels and primers for next generation sequencing studies on neurodegenerative disorders. Mol Cell Toxicol 11, 89 (2015)
Giau, V. V., Pyun, J. M., Bagyinszky, E., An, S. S. A. & Kim, S. Y. A pathogenic PSEN2 p.His169Asn mutation associated with early-onset Alzheimer’s disease. Clin In-terv Aging 13, 1321–1329 (2018).
Perry, D. C. et al. Progranulin mutations as a risk factor for Alzheimer’s Disease. JAMA Neurology 70, 774–778 (2013).
Piaceri, I. et al. Novel GRN Mutations in Alzheimer’s Disease and Frontotemporal Lobar Degeneration. J Alz-heimers Dis 62, 1683–1689 (2018).
Källberg, M. et al. Template-based protein structure modeling using the Raptor X web server. Nature Protocols 7, 1511–1522 (2012).
McDaniel, L. D., Lukovits, T. & McDaniel, K. D. Alzheimer’s disease: The problem of incorrect clinical diagnosis. J Geriatr Psychiatry Neurol 6, 230–234 (1993).
van der Zee, J., Sleegers, K. & Van Broeckhoven, C. Invited article: The Alzheimer disease-frontotemporal lobar degeneration spectrum. Neurology 71, 1191–1197 (2008).
Hutton, M. et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998).
Poorkaj, P. et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43, 815–825 (1998).
Baker, M. et al. Mutations in progranulin cause tau-neg-ative frontotemporal dementia linked to chromosome 17. Nature 442, 916–919 (2006).
Rademakers, R. et al. Phenotypic variability associated with progranulin haploinsuffciency in patients with the common 1477C→T (Arg493X) mutation: an international initiative. Hutton M Lancet Neurol 6, 857–868 (2007).
Mesulam, M. et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 63, 709–719 (2008).
Le Ber, I. et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain 131, 732–746 (2008).
Fenoglio, C. et al. Rs5848 variant influences GRN mRNA levels in brain and peripheral mononuclear cells in patients with Alzheimer’s disease. J Alzheimers Dis 18, 603–612 (2009).
Lee, M. J., Chen, T. F., Cheng, T. W. & Chiu, M. J. rs5848 variant of progranulin gene is a risk of Alzheimer’s disease in the Taiwanese population. Neurodege-ner Dis 8, 216–220 (2011).
Viswanathan, J. et al. An association study between granulin gene polymorphisms and Alzheimer’s disease in Finnish population. Am J Med Genet B Neuropsychi-atr Genet 150B, 747–750 (2009).
Bit-Ivan, E. N. et al. A Novel GRN Mutation (GRN c.708+6_+9delTGAG) in Frontotemporal Lobar Degeneration with TDP-43-Positive Inclusions: Clinicopatho-logic Report of 6 Cases. J Neuropathol Exp Neurol 73, 467–473 (2014).
Rizzu, P. et al. High prevalence of mutations in the mi-crotubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am J Hum Genet 64, 414–421 (1999).
Le Ber, I. et al. Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat 28, 846–855 (2007).
Gass, J. et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 15, 2988–3001 (2006).
Gijselinck, I., Van Broeckhoven, C. & Cruts, M. Gran-ulin mutations associated with frontotemporal lobar degeneration and related disorders: An update. Hum Mutat 29, 1373–1386 (2009).
Finch, N. et al. Plasma progranulin levels predict pro-granulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 132, 583–591 (2009).
Bruni, A. C. et al. Heterogeneity within a large kindred with frontotemporal dementia: a novel progranulin mutation. Neurology 69, 140–147 (2007).
Giau, V. V., Bagyinszky, E., An, S. S. & Kim, S. Y. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr Dis Treat 11, 1723–1737 (2015).
Giau, V. V., Lee, H., Shim, K. H., Bagyinszky, E. & An, S. S. A. Genome-editing applications of CRISPR-Cas9 to promote in vitro studies of Alzheimer’s disease. Clin Interv Aging 13, 221–233 (2018).
Van Giau, V. & An, S. S. Emergence of exosomal miR-NAs as a diagnostic biomarker for Alzheimer’s disease. J Neurol Sci 15, 141–152 (2016).
Giau, V. V. et al. Gut Microbiota and Their Neuroinfam-matory Implications in Alzheimer’s Disease. Nutrients 10, 1765 (2018). doi: 10.3390/nu10111765.
Van Giau, V. et al. Identifcation of a novel mutation in APP gene in a Thai subject with early-onset Alzheimer’s disease. Neuropsychiatr Dis Treat 14, 3015–3023 (2018).
Bagyinszky, E. et al. PSEN1 p.Thr116Ile Variant in Two Korean Families with Young Onset Alzheimer’s Disease. Int J Mol Sci 19, 2604 (2018). doi: 10.3390/ijms19092604.
Giau, V. V. et al. Novel PSEN1 p.Gly417Ala mutation in a Korean patient with early-onset Alzheimer’s disease with parkinsonism. Neurobiol Aging 72, 188.e13–188.e17 (2018).
Van Giau, V. et al. Identifcation of a novel mutation in APP gene in a Thai subject with early-onset Alzheimer’s disease. Neuropsychiatr Dis Treat 14, 3015–3023 (2018).
Acknowledgements
This research was supported by a National Research Foundation of Korea (NRF) Grants awarded by the Korean government (MEST, No. 2017R1A2B 4012636 & 2017R1C1B5017807). The authors would like to express their gratitude to the proband patient and his family members for their time and support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interest
Lingyan Shen, Seong Soo A. An, Eva Bagyinszky, Vo Van Giau, Seong Hye Choi, SangYun Kim declares that they have no conflicts of interest.
Human and animal rights
All human DNA samples were approved by the Institutional Review Board of the Seoul National University Bundang Hospital and Inha University Hospital.
Rights and permissions
About this article
Cite this article
Shen, L., An, S.S.A., Bagyinszky, E. et al. Novel GRN mutations in Koreans with Alzheimer’s disease. Mol. Cell. Toxicol. 15, 345–352 (2019). https://doi.org/10.1007/s13273-019-0038-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-019-0038-4