Abstract
Backgrounds
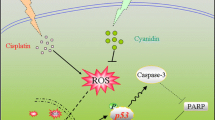
Oxaliplatin is a chemotherapeutic agent that induces neuropathy through unknown mechanisms and therefore, its pharmacological performance is limited. Silymarin, a well-known hepatoprotective natural flavonoid mixture, has neuroprotective effects against certain neurodegenerative or neurotoxic stimuli.
Methods
We tested whether silymarin protects against oxaliplatin-induced neurotoxicity by using a neuronal cell culture system. Using differentiated SH-SY5Y cells, effects of silymarin on the oxaliplatin-mediated cytotoxicity for cell viability, oxidative stress and BDNF expression.
Results
Treatment of neuronal cells with oxaliplatin decreased cell viability, which was accompanied by increase in levels of the apoptotic marker cleaved poly-(ADP-ribose) polymerase (PARP) and malondialdehyde (MDA), a marker of lipid peroxidation. We found that oxaliplatin-induced cell death was partially mediated by p38-MAPK activation, which was significantly inhibited by silymarin. Silymarin slightly but not significantly inhibited oxaliplatin-induced oxidative stress. It also upregulated brain-derived neurotrophic factor (BDNF) expression and increased calcium-calmodulin kinase II and CREB activities. The observation of cell morphology revealed that silymarin induced dendritic outgrowth, which was validated by the increased expression of β-III tubulin protein. Furthermore, we observed that oxaliplatin-induced loss of dendritic outgrowth and BDNF downregulation were partially blocked by silymarin.
Conclusion
Our results suggested that oxaliplatin-induced neuropathy may be caused by combined mechanisms of increased oxidative stress, p38 MAPK-mediated apoptosis, and reduction of BDNF expression. All these changes were significantly inhibited by silymarin.
Similar content being viewed by others
References
Goldberg, R. M. et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22, 23–30 (2004).
Wang, D. & Lippard, S. J. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4, 307–320 (2005).
Land, S. R. et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSAABP C-7. J Clin Oncol 25, 2205–2211 (2007).
Carozzi, V. A., Canta, A. & Chiorazzi, A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms?. Neurosci Lett 596, 90–107 (2015).
Post-White, J., Ladas, E. J. & Kelly, K. M. Advances in the use of milk thistle (silybummarianum). Integr Cancer Ther 6, 104–109 (2007).
Loguercio, C. & Festi, D. Silybin and the liver: From basic research to clinical practice. World J Gastroenterol 17, 2288–2301 (2011)
Borah, A. et al. Neuroprotective potential of silymarin against CNS disorders: insight into the pathways and molecular mechanisms of action. CNS Neurosci Ther 19, 847–853 (2013).
Marchetti, P. et al. Apoptosis induced by oxaliplatin in human colon cancer HCT15 cell line. Anticancer Res 24, 219–226 (2004).
Tan, S., Peng, X., Peng, W., Zhao, Y. & Wei, Y. Enhancement of oxaliplatin-induced apoptosis and tumor suppression by 30methyladenine in colon cancer. Oncol Lett 9, 2056–2062 (2015).
Agarwal, R., Agarwal, C., Ichikawa, H., Singh, R. P. & Aggarwal, B. B. Anticancer Potential of silymarin: From Bench to bed side. Anticancer Res 26, 4457–4498 (2006).
Vue, B. & Chen, Q. H., The potential of flavonolignans in prostate cancer management. Curr Med Chem 23, 3925–3950 (2016).
Khorsandi, L., Saki, G., Bavarsad, N. & Mombeini, M. Silymarin induces a multi-targeted cell death process in the human colon cancer cell line HT-29. Biomed Phrmacother 94, 890–897 (2017).
Kittur, S. et al. Neurotrophic and neuroprotective effects of milk thistle (silybummarianum) on neurons in culture. J Mol Neurosci 18, 265–269 (2002).
Lonze, B. E. & Ginty, D. D. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623 (2002).
Yan, X. et al. CaMKII-mediated CREB phosphorylation is involved in Ca2+-induced BDNF mRNA transcription and neurite outgrowth promoted by electrical stimulation. PloS One 11, e0162784, Doi:10.1371/journal.pone.0162784.
Surai, P. F. Silymarin as a natural antioxidant: An Overview of the current evidence and perspectives. Antioxidants (Basel) 4, 204–247 (2015).
Canals, J. M. et al. Expression of brain-derived neurotrophic factor in cortical neurons is regulated by striatal target area. J Cell Physiol 213, 341–347 (2001).
Figurov, A., Pozzo-Miller, L. D., Olafsson, P., Wang, T. & Lu, B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381, 706–709 (1996).
Abel, T. et al. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based longterm memory. Cell 88, 615–626 (1997)
Hu, Y., Liu, M. Y., Liu, P., Dong, X. & Boran, A. D. Neuroprotective effects of 3,6′-disinapoyl sucrose through increased BDNF levels and CREB phosphorylation via the CaMKII and ERK1/2 pathway. J Mol Neurosci 54, 600–607 (2014).
Velasco, R. et al. Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry 85, 392–398 (2014).
Park, S. B. et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 63, 419–437 (2013).
Huang, Z. Z. et al. Cerebrospinal fluid oxaliplatincontribuetes to the acute pain induced by systemic administration of oxaliplatin. Anesthesiology 124, 1109–1121 (2016).
Massicot, F. et al. P2X7 cell death receptor activation and mitochondrial impairment in oxliplatin-induced apoptosis and neuronal injury: Cellular mechanisms and in vivo approach. PLoS One 8, e66830, Doi:10.1371/journal.pone.006830 (2013).
Xiao, W. H. & Bennett, G. J. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain 153, 704–709 (2012).
Chocry, M., Leloup, L. & Kovacic, H. Reversion of resistance to oxaliplatin by inhibition of p38 MAPK in colorectal cancer cell lines: involvement of the calpain/Nox1 pathway. Oncotarget 8, 103710–103730 (2017).
Liu, H. F., Hu, H. C. & Chao, J. I. Oxaliplatin down-regulates survivin by p38 MAP kinase and proteasome in human colon cancer cells. Chem Biol Interact 188, 535–545 (2010).
Dalrymple, S. A. p38 mitogen activated protein kinase as a therapeutic target for Alzheimer’s disease. J Mol Neurosci 19, 295–299 (2002).
Hensley, K. et al. P38 kinase is activated in the Alzheimer’s disease brain. J Neurochem 72, 2053–2058 (1999).
Feijoo, C., Campbell, D. G., Jakes, R., Goedert, M. & Cuenda, A. Evidence that phosphorylation of the microtubule-associated protein Tau by SAPK4/p38delta at Thr50 promotes microtubule assembly. J Cell Sci 118, 397–408 (2005).
Goedert, M. et al. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Lett 409, 57–62 (1997).
Jenkins, S. M., Zinnerman, M., Garner, C. & Johnson, G. V. Modulation of tau phosphorylation and intracellular localization by cellular stress. Biochem J 345, 263–270 (2000)
Criscuolo, C., Fabiani, C., Bonadonna, C., Origlia, N. & Domenici, L. BDNF prevents amyloid-dependent impairment of LPT in the entorhinal cortex by attenuating p38 MAPK phosphorylation. Neurobiol Aging 36, 1303–1309 (2015).
Li, Y., Liu, L., Barger, S. W. & Griffin, W. S. Interleukin-1 mediates pathological effects of microglia on Tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci 23, 1605–1611 (2003).
Li, Y., Jiang, B., Ensign, W. Y., Vogt, P. K. & Han, J. Myogenic differentiation requires signaling through both phosphatidylinositol 3-kinase and p38 MAP kinase. Cell Signal 12, 751–757 (2000).
Zester, A., Gredinger, E. & Bengal, E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J Biol Chem 274, 5193–5200 (1999).
Eckert, R. L. et al. p38 Mitogen-activated protein kinases on the body surface-A function for p38 delta. J Invest Dermatol 120, 823–828 (2003).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Choi, JY., Yi, H.G., Park, CS. et al. Inhibition of oxaliplatin-induced neurotoxicity by silymarin through increased expression of brain-derived neurotrophic factor and inhibition of p38-MAPK. Mol. Cell. Toxicol. 15, 145–152 (2019). https://doi.org/10.1007/s13273-019-0018-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-019-0018-8