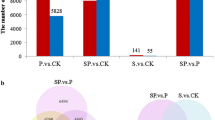
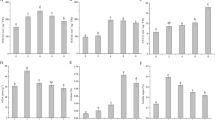
Abstract
Hydrogen sulfide (H2S), a small bioactive gas, has been proved functioning in plant growth and development as well as alleviation of abiotic stresses, which including promoting seed germination, accelerating embryonic root growth, regulating flower senescence, inducing stomatal closure, and defending drought, heat, heavy metals and osmotic stresses etc. However, the molecular functioning mechanism of H2S was still unclear. The primary objective of this research was to analyze the transcriptional differences and functional genes involved in the H2S responses. In details, 4-week-old plantlets in tissue culture of grapevine (Vitis vinifera L.) cultivar ‘Zuoyouhong’ were sprayed with 0.1 mM NaHS for 12 h, and then transcriptome sequencing and qRT-PCR analysis were used to study the transcriptional differences and functional genes involved in the H2S responses. Our results indicated that 650 genes were differentially expressed after H2S treatment, in which 224 genes were up-regulated and 426 genes were down-regulated. The GO enrichment analysis and KEGG enrichment analysis results indicated that the up-regulated genes after H2S treatment focused on carbon metabolism, biosynthesis of amino acids, and glycolysis/gluconeogenesis, and the down-regulated genes were mainly in metabolic pathways, biosynthesis of secondary metabolites, and plant hormone signal transduction. Analyzing the transcription factor coding genes in details, it was indicated that 10 AP2/EREBPs, 5 NACs, 3 WRKYs, 3 MYBs, and 2 bHLHs etc. transcription factor coding genes were up-regulated, while 4 MYBs, 3 OFPs, 3 bHLHs, 2 AP2/EREBPs, 2 HBs etc. transcription factor coding genes were down-regulated. Taken together, H2S increased the productions in secondary metabolites and a variety of defensive compounds to improve plant development and abiotic resistance, and extend fruits postharvest shelf life by regulating the expression of AP2/EREBPs, WRKYs, MYBs, CABs, GRIP22, FERRITINs, TPSs, UGTs, and GHs etc.






Similar content being viewed by others
References
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15(1):63–78
Agarwal M, Hao Y, Kapoor A, Dong C, Fujii H, Zheng X, Zhu J (2006) A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem 281(49):37636–37645
Ali B, Gill RA, Yang S, Gill MB, Ali S, Rafiq MT, Zhou W (2014) Hydrogen sulfide alleviates cadmium-induced morpho-physiological and ultrastructural changes in Brassica napus. Ecotoxicol Environ Saf 110:197–207
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11(10):R106
Briat JF, Ravet K, Arnaud N, Duc C, Boucherez J, Touraine B, Cellier F, Gaymard F (2010) New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot 105:811–822
Chen J, Wu FH, Shang YT, Wang WH, Hu WJ, Simon M, Liu X, Shangguan ZP, Zheng HL (2015a) Hydrogen sulfide improves adaptation of Zea mays seedlings to iron deficiency. J Exp Bot 66(21):6605–6622
Chen J, Wang WH, Wu FH, He EM, Liu X, Shangguan ZP, Zheng HL (2015b) Hydrogen sulfide enhances salt tolerance through nitric oxide-mediated maintenance of ion homeostasis in barley seedling roots. Sci Rep 5:12516
Chen J, Shang YT, Wang WH, Chen XY, He EM, Zheng HL, Shangguan Z (2016) Hydrogen sulfide-mediated polyamines and sugar changes are involved in hydrogen sulfide-induced drought tolerance in Spinacia oleracea seedlings. Front Plant Sci 7:1173
Chen Z, Chen M, Jiang M (2017) Hydrogen sulfide alleviates mercury toxicity by sequestering it in roots or regulating reactive oxygen species productions in rice seedlings. Plant Physiol Biochem 111:179–192
Cheng W, Zhang L, Jiao C, Su M, Yang T, Zhou L, Peng R, Wang R, Wang C (2013) Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiol Biochem 70:278–286
Christou A, Manganaris GA, Papadopoulos I, Fotopoulos V (2013) Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot 64:1953–1966
Davies C, Robinson SP (2000) Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening. Cloning and characterization of cDNAs encoding putative cell wall and stress response proteins. Plant Physiol 122:803–812
Garcia-Mata C, Lamattina L (2010) Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol 188:977–984
Grubb CD, Zipp BJ, Ludwig-muller J, Masuno MN, Molinski TF, Abel S (2004) Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J 40(6):893–908
Guo C, Guo R, Xu X, Gao M, Li X, Song J, Zheng Y, Wang X (2014) Evolution and expression analysis of the grape (Vitis vinifera L.) WRKY gene family. J Exp Bot 65:1513–1528
Gutterson N, Reuber TL (2004) Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol 7:465–471
Harrington HM, Smith IK (1980) Cysteine metabolism in cultured tobacco cells. Plant Physiol 65:151–155
Hossain MA, Ku ZG, Hoque TS, Burritt DJ, Fujita M, Munne-Bosch S (2018) Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: key regulators and possible mechanisms. Protoplasma 255(1):399–412
Hou Z, Liu J, Hou L, Li X, Liu X (2011) H2S may function downstream of H2O2 in jasmonic acid-induced stomatal closure in Vicia faba. Chin Bull Bot 46:396–406
Jansson S (1999) A guide to the identification of the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci 4:236–240
Jin Z, Xue S, Luo Y, Tian B, Fang H, Li H, Pei Y (2013) Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol Biochem 62:41–46
Katiyar A, Smita S, Lenka SK, Rajwanshi R, Chinnusamy V, Bansal KC (2012) Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis. BMC Genomics 13(1):544
Khan MN, Mobin M, Abbas ZK, Siddiqui MH (2017) Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 68:91–102
Kharbech O, Houmani H, Chaoui A, Corpas FJ (2017) Alleviation of Cr(VI)-induced oxidative stress in maize (Zea mays L.) seedlings by NO and H2S donors through differential organ-dependent regulation of ROS and NADPH-recycling metabolisms. J Plant Physiol 219:71–80
Kushnir S, Babiychuk E, Storozhenko S, Davey MW, Papenbrock J, De Rycke R, Engler G, Stephan UW, Lange H, Kispal G, Lill R, Van Montagu M (2001) A mutation of the mitochondrial ABC transporter stat1 leads to dwarfism and chlorosis in the Arabidopsis mutant starik. Plant Cell 13:89–100
Lai D, Mao Y, Zhou H, Li F, Wu M, Zhang J, He Z, Cui W, Xie Y (2014) Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Sci 225:117–129
Latchman DS (1997) Transcription factors: an overview. Int J Biochem B 29(12):1305–1312
Leon S, Touraine B, Briat JF, Lobreaux S (2002) The AtNFS2 gene from Arabidopsis thaliana encodes a Nifs-like plastidial cysteine desulphurase. Biochem J 366:557–564
Li ZG, Ding XJ, Du PF (2013) Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Physiol 170:741–747
Ma Q, Zhang G, Hou L, Wang W, Hao J, Liu X (2015) Vitis vinifera VvWRKY13 is an ethylene biosynthesis-related transcription factor. Plant Cell Rep 34(9):1593–1603
Mancardi D, Penna C, Merlino A, DelSoldato P, Wink DA, Pagliaro P (2009) Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochim Biophys Acta 1787:864–872
Marchive C, Mzid R, Deluc L, Barrieu F, Pirrello J, Gauthier A, Corio Costet MF, Regad F, Cailleteau B, Hamdi S (2007) Isolation and characterization of a Vitis vinifera transcription factor, VvWRKY1, and its effect on responses to fungal pathogens in transgenic tobacco plants. J Exp Bot 58:1999–2010
Marchive C, Leon C, Kappel C, Coutos-Thevenot P, Corio-Costet MF, Delrot S, Lauvergeat V (2013) Over-expression of VvWRKY1 in grapevines induces expression of jasmonic acid pathway-related genes and confers higher tolerance to the downy mildew. PLoS ONE 8:e54185
Mei Y, Chen H, Shen W, Shen W, Huang L (2017) Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol 17(1):162
Merz RP, Moser T, Holl J, Kortekamp A, Buchholz G, Zyprian E, Bogs J (2015) The transcription factor VvWRKY33 is involved in the regulation of grapevine (Vitis vinifera) defense against the oomycete pathogen Plasmopara viticola. Physiol Plant 153:365–380
Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2011) AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta 1819:86–96
Mostofa MG, Rahman A, Ansary MMU, Watanabe A, Fujita M, Tran LS (2015) Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci Rep 5:14078
Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140:411–432
Ni ZJ, Hu KD, Song CB, Ma RH, Li ZR, Zheng JL, Fu LH, Wei ZJ, Zhang H (2016) Hydrogen sulfide alleviates postharvest senescence of grape by modulating the antioxidant defenses. Oxid Med Cell Longev 8:4715651
Papenbrock J, Riemenschneider A, Kamp A, Schulz-Vogt HN, Schmidt A (2007) Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants—from the field to the test tube and back. Plant Biol 9:582–588
Peng X, Liu H, Wang D, Shen S (2016) Genome-wide identification of the Jatropha curcas MYB family and functional analysis of the abiotic stress responsive gene JcMYB2. BMC Genomics 17:251
Priest DM, Ambrose SJ, Vaistij FE, Elias L, Higgins GS, Ross AR, Abrams SR, Bowles D (2006) Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana. Plant J 46:492–502
Ravet K, Touraine B, Boucherez J, Briat JF, Gaymard F, Cellier F (2009) Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J 57:400–412
Rivero RM, Mestre TC, Mittler R, Rubio F, Garcia-Sanchez F, Martinez V (2014) The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ 37(5):1059–1073
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290:998–1009
Schmidt A (1982) A cysteine desulfhydrase from spinach leaves specific for d-cysteine. Z Pflanzenphysiol 107:301–312
Schwartz E, Stasys R, Aebersold R, McGrath JM, Green BR, Pichersky E (1991) Sequence of a tomato gene encoding a third type of LHCII chlorophyll a/b-binding polypeptide. Plant Mol Biol 17:923–925
Scuffi D, Alvarez C, Laspina N, Gotor C, Lamattina L, Garcia-Mata C (2014) Hydrogen sulfide generated by l-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol 166(4):2065–2076
Shan C, Zhang S, Ou X (2018) The roles of H2S and H2O2 in regulating AsA-GSH cycle in the leaves of wheat seedlings under drought stress. Protoplasma. https://doi.org/10.1007/s00709-018-1213-5
Shi H, Ye T, Chan Z (2013) Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol Biochem 71:226–234
Silva J, Kim YJ, Sukweenadhi J, Rahimi S, Kwon WS, Yang DC (2016) Molecular characterization of 5-chlorophyll a/b-binding protein genes from Panax ginseng Meyer and their expression analysis during abiotic stresses. Photosynthetica 54(3):446–458
Tai CH, Cook PF (2000) O-Acetylserine sulfhydrylase. Adv Enzymol Relat Areas Mol Biol 74:185–234
Toit AD (2015) METABOLISM: the health benefits of hydrogen sulphide. Nat Rev Mol Cell Biol 16:68
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515
Wang J, Ma XM, Kojima M, Sakakibara H, Hou BK (2011) N-Glucosyltransferase UGT76C2 is involved in cytokinin homeostasis and cytokinin response in Arabidopsis thaliana. Plant Cell Physiol 52(12):2200–2213
Wang RK, Cao ZH, Hao YJ (2014) Overexpression of a R2R3 MYB gene MdSIMYB1 increases tolerance to multiple stresses in transgenic tobacco and apples. Physiol Plant 150:76–87
Xie Y, Zhang C, Lai D, Sun Y, Samma MK, Zhang J, Shen W (2014) Hydrogen sulfide delays GA triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J Plant Physiol 171:53–62
Yadav K, Patel P, Srivastava AK, Ganapathi TR (2017) Overexpression of native ferritin gene MusaFer1 enhances iron content and oxidative stress tolerance in transgenic banana plants. PLoS ONE 12(11):e0188933
Young MD, Wakefield MJ, Smyth GK, Oshlack A (2010) Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 11(2):R14
Zang X, Geng X, Wang F, Liu Z, Zhang L, Zhao Y, Tian X, Ni Z, Yao Y, Xin M, Hu Z, Sun Q, Peng H (2017) Overexpression of wheat ferritin gene TaFER-5B enhances tolerance to heat stress and other abiotic stresses associated with the ROS scavenging. BMC Plant Biol 17:14
Zarei A, Korbes AP, Younessi P, Montiel G, Champion A, Memelink J (2011) Two GCC boxes and AP2/ERF-domain transcription factor ORA59 in jasmonate/ethylene-mediated activation of the PDF1.2 promoter in Arabidopsis. Plant Mol Biol 75:321–331
Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo JP (2008) Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50:1518–1529
Zhang H, Hu SL, Zhang ZJ, Hu LY, Jiang CX, Wei ZJ, Liu J, Wang HL, Jiang ST (2011) Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol Technol 60:251–257
Zheng Y, Jiao C, Sun H, Rosli HG, Pombo MA, Zhang P, Banf M, Dai X, Martin GB, Giovannoni JJ, Zhao PX, Rhee SY, Fei Z (2016) iTAK: a program for genome-wide prediction and classification of plant transcription factors, transcriptional regulators, and protein kinases. Mol Plant 9:1667–1670
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Acknowledgements
This work was supported by Science and Technology Project of Higher Education in Shandong Province (Grant No. J14LE12), Research Foundation for Advanced Talents of Qingdao Agricultural University, and National Natural Science Foundation of China (Grant No. 31540090).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declared that the authors of this paper have no conflict of interest.
Ethical approval
This article does not contain any studies with human subjects or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1: The grapevine gene annotation information in this research. (XLS 41196 KB)
13258_2018_723_MOESM4_ESM.tif
Supplementary material 4: The FPKM density distribution of H2S treated (H2S) and untreated grapevine plantlets in tissue culture (CTR). In figure, the X-axis number was calculated by log10(FPKM+1), and the Y-axis represented the gene density. (TIF 89 KB)
13258_2018_723_MOESM5_ESM.tif
Supplementary material 5: The volcano plot of differentially expressed genes with adjusted p-value <0.05 after H2S treatment. The X-axis indicated the log fold change of genes after H2S treatment, and the Y-axis showed the significant differences of gene expression. The highly expressed genes were represented by red spots, and the lowly regulated genes were denoted by green spots in the figure. (TIF 90 KB)
13258_2018_723_MOESM6_ESM.tif
Supplementary material 6: The volcano plot of differentially expressed genes with adjusted p-value <0.05, fold change >2, and the average FPKM >1 after H2S treatment. The highly expressed genes were panted by red color, and the lowly regulated genes were painted by green. (TIF 97 KB)
13258_2018_723_MOESM10_ESM.xls
Supplementary material 10: The KEGG annotation of up-regulated DEGs used for functional enrichment analysis. (XLS 58 KB)
13258_2018_723_MOESM11_ESM.xls
Supplementary material 11: The GO annotation of down-regulated DEGs used for functional enrichment analysis. (XLS 456 KB)
13258_2018_723_MOESM12_ESM.xls
Supplementary material 12: The KEGG annotation of down-regulated DEGs used for functional enrichment analysis. (XLS 54 KB)
Rights and permissions
About this article
Cite this article
Ma, Q., Yang, J. Transcriptome profiling and identification of functional genes involved in H2S response in grapevine tissue cultured plantlets. Genes Genom 40, 1287–1300 (2018). https://doi.org/10.1007/s13258-018-0723-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-018-0723-z