Abstract
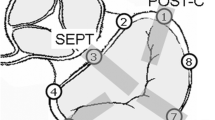
To determine how extracellular matrix and contractile valvular cells contribute to the heterogeneous motion and strain across the mitral valve (MV) during the cardiac cycle, regional MV material properties, matrix composition, matrix turnover, and cell phenotype were related to regional leaflet strain. Radiopaque markers were implanted into 14 sheep to delineate the septal (SEPT), lateral (LAT), and anterior and posterior commissural leaflets (ANT-C, POST-C). Videofluoroscopy imaging was used to calculate radial and circumferential strains. Mechanical properties were assessed using uniaxial tensile testing and micropipette aspiration. Matrix composition and cell phenotypes were immunohistochemically evaluated within each leaflet region [basal leaflet (BL), mid-leaflet (ML), and free edge]. SEPT-BL segments were stiffer and stronger than other valve tissues, while LAT segments demonstrated more extensibility and strain. Collagens I and III in SEPT were greater than in LAT, although LAT showed greater collagen turnover [matrix metalloprotease (MMP)-13, lysyl oxidase] and cell activation [smooth muscle alpha-actin (SMaA), and non-muscle myosin (NMM)]. MMP13, NMM, and SMaA were strongly correlated with each other, as well as with radial and circumferential strains in both SEPT and LAT. SMaA and MMP13 in POST-C ML was greater than ANT-C, corresponding to greater radial strains in POST-C. This work directly relates leaflet strain, material properties, and matrix turnover, and suggests a role for myofibroblasts in the heterogeneity of leaflet composition and strain. New approaches to MV repair techniques and ring design should preserve this normal coupling between leaflet composition and motion.







Similar content being viewed by others
References
Balachandran, K., S. Konduri, P. Sucosky, H. Jo, and A. P. Yoganathan. An ex vivo study of the biological properties of porcine aortic valves in response to circumferential cyclic stretch. Ann. Biomed. Eng. 34:1655–1665, 2006.
Balachandran, K., P. Sucosky, H. Jo, and A. P. Yoganathan. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am. J. Physiol. Heart Circ. Physiol. 296:H756–H764, 2009.
Carew, E. O., and I. Vesely. A new method of estimating gauge length for porcine aortic valve test specimens. J. Biomech. 36:1039–1042, 2003.
Chaput, M., M. D. Handschumacher, F. Tournoux, L. Hua, J. L. Guerrero, G. J. Vlahakes, et al. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation. 118:845–852, 2008.
Chiechi, M. A., W. M. Lees, and R. Thompson. Functional anatomy of the normal mitral valve. J. Thorac. Surg. 32:378–398, 1956.
Cochran, R. P., K. S. Kunzelman, C. J. Chuong, M. S. Sacks, and R. C. Eberhart. Nondestructive analysis of mitral valve collagen fiber orientation. ASAIO Trans. 37:M447–M448, 1991.
Gheewala, N., K. A. Schwarz, and K. J. Grande-Allen. Organ culture of porcine mitral valves as a novel experimental paradigm. Cardiovasc. Eng. Technol. 4:139–150, 2013.
Grashow, J. S., M. S. Sacks, J. Liao, and A. P. Yoganathan. Planar biaxial creep and stress relaxation of the mitral valve anterior leaflet. Ann. Biomed. Eng. 34:1509–1518, 2006.
Grashow, J. S., A. P. Yoganathan, and M. S. Sacks. Biaixal stress-stretch behavior of the mitral valve anterior leaflet at physiologic strain rates. Ann. Biomed. Eng. 34:315–325, 2006.
Grigioni, F., M. Enriquez-Sarano, K. J. Zehr, K. R. Bailey, and A. J. Tajik. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 103:1759–1764, 2001.
Gupta, V., J. A. Werdenberg, J. S. Mendez, and K. Jane Grande-Allen. Influence of strain on proteoglycan synthesis by valvular interstitial cells in three-dimensional culture. Acta Biomater. 4:88–96, 2008.
Karlsson, M. O., J. R. Glasson, A. F. Bolger, G. T. Daughters, M. Komeda, L. E. Foppiano, et al. Mitral valve opening in the ovine heart. Am. J. Physiol. 274:H552–H563, 1998.
Krishnamurthy, V. K., F. Guilak, D. A. Narmoneva, and R. B. Hinton. Regional structure-function relationships in mouse aortic valve tissue. J. Biomech. 44:77–83, 2011.
Ku, C.-H., P. H. Johnson, P. Batten, P. Sarathchandra, R. C. Chambers, P. M. Taylor, et al. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc. Res. 71:548–556, 2006.
Kunzelman, K. S., and R. P. Cochran. Stress/strain characteristics of porcine mitral valve tissue: parallel versus perpendicular collagen orientation. J. Card. Surg. 7:71–78, 1992.
Kunzelman, K. S., R. P. Cochran, S. S. Murphree, W. S. Ring, E. D. Verrier, and R. C. Eberhart. Differential collagen distribution in the mitral valve and its influence on biomechanical behaviour. J. Heart Valve Dis. 2:236–244, 1993.
Liao, J., L. Yang, J. Grashow, and M. S. Sacks. The relation between collagen fibril kinematics and mechanical properties in the mitral valve anterior leaflet. J. Biomech. Eng. 129:78–87, 2007.
Merryman, W. D., H. Y. Shadow Huang, F. J. Schoen, and M. S. Sacks. The effects of cellular contraction on aortic valve leaflet flexural stiffness. J. Biomech. 39:88–96, 2006.
Merryman, W. D., I. Youn, H. D. Lukoff, P. M. Krueger, F. Guilak, R. A. Hopkins, et al. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am. J. Physiol. Hear. Circ. Physiol. 290:H224–H231, 2006.
Messier, R. H., B. L. Bass, H. M. Aly, J. L. Jones, P. W. Domkowski, R. B. Wallace, et al. Dual structural and functional phenotypes of the porcine aortic valve interstitial population: characteristics of the leaflet myofibroblast. J. Surg. Res. 57:1–21, 1994.
Rabkin, E., M. Aikawa, J. R. Stone, Y. Fukumoto, P. Libby, and F. J. Schoen. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 104:2525–2532, 2001.
Sacks, M. S., Y. Enomoto, J. R. Graybill, W. D. Merryman, A. Zeeshan, A. P. Yoganathan, et al. In-vivo dynamic deformation of the mitral valve anterior leaflet. Ann. Thorac. Surg. 82:1369–1377, 2006; (Elsevier).
Stephens, E. H., N. de Jonge, M. P. McNeill, C. A. Durst, and K. J. Grande-Allen. Age-related changes in material behavior of porcine mitral and aortic valves and correlation to matrix composition. Tissue Eng. Part A. 16:867–878, 2010.
Stephens, E. H., T. C. Nguyen, A. Itoh, N. B. Ingels, D. C. Miller, and K. J. Grande-Allen. The effects of mitral regurgitation alone are sufficient for leaflet remodeling. Circulation. 118:S243–S249, 2008.
Timek, T. A., P. Dagum, D. T. Lai, D. Liang, G. T. Daughters, F. Tibayan, et al. Tachycardia-induced cardiomyopathy in the ovine heart: mitral annular dynamic three-dimensional geometry. J. Thorac. Cardiovasc. Surg. 125:315–324, 2003.
Tsakiris, A. G., D. A. Gordon, Y. Mathieu, and L. Irving. Motion of both mitral valve leaflets: a cineroentgenographic study in intact dogs. J. Appl. Physiol. 39:359–366, 1975.
Tsakiris, A. G., G. Von Bernuth, G. C. Rastelli, M. J. Bourgeois, J. L. Titus, and E. H. Wood. Size and motion of the mitral valve annulus in anesthetized intact dogs. J. Appl. Physiol. 30:611–618, 1971.
Walmsley, R. Anatomy of human mitral valve in adult cadaver and comparative anatomy of the valve. Br Hear. J. 40:351–366, 1978.
Wang, J., H. Chen, A. Seth, and C. A. McCulloch. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 285:H1871–H1881, 2003.
Zhao, R., K. L. Sider, and C. A. Simmons. Measurement of layer-specific mechanical properties in multilayered biomaterials by micropipette aspiration. Acta Biomater 7:1220–1227, 2011; (Acta Materialia Inc.).
Acknowledgments
The authors thank all the members of the Grande-Allen lab, especially Indrajit Nandi. The authors also appreciate the statistical expertise of Dr. Scott Baggett and use of the micropipette aspiration equipment from Dr. Robert Raphael.
Funding
Funding for this project came in part from graduate fellowships from the Hertz Foundation (EHS), National Institutes of Health (F30HL094019, EHS) and American Heart Association (13PRE14110003, PSC) and National Institutes of Health grants R01HL067025 and R01HL029589 (DCM).
Conflict of interest
Elizabeth H. Stephens was supported by individual graduate fellowships from the Hertz Foundation and NIH F30HL094019. Patrick S. Connell was supported by an individual fellowship from the American Heart Association 13PRE14110003. D. Craig Miller was supported by NIH R01 HL067025 and R01 HL029589. K. Jane Grande-Allen has served as a consultant for Edwards Lifesciences. Monica M. Fahrenholtz, Tomasz A. Timek, George T. Daughters, Joyce J. Kuo, Aaron M. Patton, Neil B. Ingels, Jr. have no conflict of interest.
Human Subjects Declaration
No human subjects studies were carried out by the authors for this article.
Animal Studies Declaration
The animal studies involved in this article were performed according to protocols approved by the Stanford University and Rice University Institutional Animal Care and Use Committees.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Karyn Kunzelman oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Stephens, E.H., Connell, P.S., Fahrenholtz, M.M. et al. Heterogeneity of Mitral Leaflet Matrix Composition and Turnover Correlates with Regional Leaflet Strain. Cardiovasc Eng Tech 6, 141–150 (2015). https://doi.org/10.1007/s13239-015-0214-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-015-0214-1