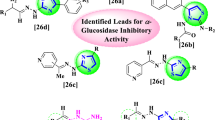
Abstract
Phenylthiourea was synthesized for the first time with a yield of 75–80% based on thiourea, in the presence of various catalysts, and optimal conditions were identified for some reactions. At the same time, the condensation of their methylene-active molecules and various aldehydes in acidic media caused the synthesis of di-, tetra-, hexahydropyrimidinethiones, which are not known in the literature so far; the role of different catalysts in this process were comparatively studied. Synthesis of heterocyclic compounds containing phenol hydroxyl, mono-, dual-, triple-amine, -thion, and hydroxyl groups in the presence of ionic liquids, CCl3COOH CF3COOH, NiCl2.6H2O catalysts as well as in the increase of yield percentages showed that the use of environmentally and economically efficient ionic liquids among these catalysts allows to obtain purposeful compounds with the highest yield (95%). The inhibition of α-glycosidase, aldose reductase, and α-amylase enzymes by functionally substituted derivatives of thiourea and phenylthiourea (1a–1f) is then observed. Compound 1d displayed the lowest inhibitory effect against AR in these series with an IC50 value of 3.25 µM, whereas compound 1c compound displayed the highest inhibitory effect with an IC50 value of 1.46 µM. The enzymes α-amylase and α-glycosidase were also easily inhibited by these substances. All substances were examined for their capacity to inhibit the α-glycosidase enzyme, with Ki values ranging between 14.321.53 and 29.322.50 µM and IC50 values between 12.23 and 25.22 µM. Additionally, the IC50 values for the effective inhibition profile of the α-amylase, which was determined vary from 1.02 to 7.87 µM.
Graphical abstract

Enzyme inhibitor and docking of it







Similar content being viewed by others
References
G. Jenner, Effect of high pressure on Biginelli reactions. Steric hindrance and mechanistic considerations. Tetrahedron Lett. 45, 6195–6198 (2004)
J.-T. Li, J.-F. Han, J.-H. Yang, T.-S. Li, An efficient synthesis of 3,4-dihydropyrimidin-2-ones catalyzed by NH2SO3H under ultrasound irradiation. Ultrason. Sonochem. 10, 119–122 (2003)
A. Sudzhaev, R. Najafova, I. Rzayeva, Yu. Safarov, M. Allahverdiev, Study of the antioxidant properties of thiocarbamide derivatives of some amino alcohols. J. Appl. Chem. 84(8), 1329–1332 (2011)
V. Farzaliyev, A. Shuriberko, A. Sujayev, S. Osmanova, S. Gojayeva, K. Gahramanova, Synthesis, computational and biological activity of heteroatomic compounds based on phenylthiourea and acetophenone. J. Mol. Struct. 1221(9), 128844–128853 (2020)
L. Wang, C. Qian, H. Tian, M. Yun, Lanthanide triflate catalyzed one-pot synthesis of dihydropyrimidin-2(1H)-thiones by a three-component of 1,3-dicarbonyl compounds, Aldehydes, and thiourea using a solvent-free Biginelli condensation. Synth. Commun. 33, 1459–1468 (2003)
T. Laue, A. Plagens, Namen und Schlagwort-Reaktionen der Organischen Chemic. Taubner, https://www.amazon.de/Namen-Schlagwort-Reaktionen-Organischen-Teubner-Studienb%C3%BCcher/dp/3835100912 (2006)
L. Jun, Y. Bai, Z. Wang, L. Jun, B. Yang, H. Ma, One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using lanthanum chloride as a catalyst. Tetrahedron Lett. 41, 9075–9078 (2000)
S. D. Bose, M. Sudharshan, W. Ch. Sanjay, New protocol for Biginelli reaction practical synthesis of Monastrol. Arkivoc. 3, 228–236 (2005)
S. Sasaki, M. Mizuno, K. Naemura, Y. Tobe, Synthesis and anion-selective complexation of cyclophane-based cyclic thioureas. J. Org. Chem. 65, 275–283 (2000)
A. Sujayev, E. Garibov, P. Taslimi, I. Gulçin, S. Gojayeva, V. Farzaliyev, S. Alwasel, C.T. Supuran, Synthesis of some tetrahydropyrimidine-5-carboxylates, determination of their metal chelating effects and inhibition profiles against acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase. J. Enzyme Inhib. Med. Chem. 31(6), 1531–1539 (2016)
V. Farzaliyev, A. Sujayev, L. Kose, E. Garibov, İ Gülçin, V. Farzaliev, S. Alwasel, C. Supuran, Synthesis of N-Alkyl (Aril)-tetra pyrimidinethiones and investigation of their human carbonic anhydrase i and ii inhibitory effects. J. Enzyme Inhib. Med. Chem. 31(6), 1192–1197 (2016)
N. M. Emanuel, E. T. Denisov, Z. K. Maizus, V. V. Fedorova, Illustrative reactions oxidation of hydrocarbons in the liquid phase. M.: Nauka, https://search.worldcat.org/title/liquid-phase-oxidation-of-hydrocarbons/oclc/1879057 (1967)
Denisov, E.T. (1981) Rate Constants of Hemolytic Liquid-Phase Reactions. М.: Nauka, 711 (1981)
C. Bailly, Moving toward a new horizon for the aldose reductase inhibitor epalrestat to treat drug-resistant cancer. Eur J Pharmacol. 931, 175191 (2022)
R.L. Bakal, R.D. Jawarkar, J.V. Manwar, M.S. Jaiswal, A. Ghosh, A. Gandhi et al., Identification of potent aldose reductase inhibitors as antidiabetic (Anti-hyperglycemic) agents using QSAR based virtual Screening, molecular Docking, MD simulation and MMGBSA approaches. Saudi Pharmaceut. J. 30(6), 693–710 (2022)
F. Türkan, P. Taslimi, B. Cabir, M.S. Ağırtaş, Y. Erden, H.U. Celebioglu et al., Co and Zn Metal phthalocyanines with bulky substituents: anticancer, antibacterial activities and their inhibitory effects on some metabolic enzymes with molecular docking studies. Polycyclic Aromat. Compd. 42(7), 4475–4486 (2022)
P. Taslimi, F. Akhundova, M. Kurbanova, F. Türkan, B. Tuzun, A. Sujayev et al., Biological activity and molecular docking study of some bicyclic structures: antidiabetic and anticholinergic potentials. Polycyclic Aromat. Compd. 42(9), 6003–6016 (2022)
P. Taslimi, F. Türkan, D. Güngördü Solğun, A. Aras, Y. Erden, H.U. Celebioglu et al., Metal contained Phthalocyanines with 3, 4-Dimethoxyphenethoxy substituents: their anticancer, antibacterial activities and their inhibitory effects on some metabolic enzymes with molecular docking studies. J. Biomol. Struct. Dyn. 40(7), 2991–3002 (2022)
P. Taslimi, İ Gulçin, Antidiabetic potential: in vitro inhibition effects of some natural phenolic compounds on α-glycosidase and α-amylase enzymes. J. Biochem. Mol. Toxicol. 31(10), e21956 (2017)
P. Taslimi, H. Akıncıoğlu, İ Gulçin, Synephrine and phenylephrine act as α-amylase, α-glycosidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase enzymes inhibitors. J. Biochem. Mol. Toxicol. 31(11), e21973 (2017)
A. Huseynova, R. Kaya, P. Taslimi, V. Farzaliyev, Kh. Mammadyarova, A. Sujayev, B. Tüzün, U. Kocyigit, S. Alwasel, İ Gulçin, Design, synthesis, characterization, biological evaluation, and molecular docking studies of novel 1,2-aminopropanthiols substituted derivatives as selective carbonic anhydrase, acetylcholinesterase and α-glycosidase enzymes inhibitors. J. Biomol. Struct. Dyn. 40(1), 236–248 (2022)
A. Sujayev, P. Taslimi, B. Safarov, L. Aliyeva, V. Farzaliyev, R. Kaya, İ Gulçin, Synthesis, characterization and biological evaluation of N-substituted triazinane-2-thiones and theoretical–experimental mechanism of condensation reaction. Appl. Organometallic Chem. 34(2), e5329 (2020)
G.M. Morris, R. Huey, W. Lindstrom, M.F. Sanner, R.K. Belew, D.S. Goodsell, A.J. Olson, AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 30(16), 2785–2791 (2009)
B. K. Shoichet, I. D. Kuntz, Bodenhausen, G., Ernst, R. R. Prediction of binding constants for protein-ligand complexes. J. Mol. Biol. 221(2), 327–346 (1991)
O. Trott, A.J. Olson, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31(2), 455–461 (2010)
Y. Tao, Y. Zhang, Y. Cheng, Y. Wang, Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chromatogr. 27, 148–155 (2013)
P. Taslimi, C. Caglayan, İ Gulçin, The impact of some natural phenolic compounds on carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase, and α-glycosidase enzymes: an antidiabetic, anticholinergic, and antiepileptic study. J. Biochem. Mol. Toxicol. 31(12), e21995 (2017)
F. Turkan, A. Cetin, P. Taslimi, İ Gulçin, Some pyrazoles derivatives: potent carbonic anhydrase, α-glycosidase and cholinesterase enzymes inhibitors. Arch. Pharm. 351(10), 1800200 (2018)
Z. Xiao, R. Storms, A. Tsang, A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal. Biochem. 351, 146–148 (2006)
Y. Demir, L. Durmaz, P. Taslimi, İ Gülçin, Anti-diabetic properties of dietary phenolic compounds: inhibition effects on α-amylase, aldose reductase and α-glycosidase. Biotechnol. Appl. Biochem. 66(5), 781–786 (2019)
İ Gulçin, P. Taslimi, A. Aygün, N. Sadeghian, E. Bastem, O.I. Kufrevioglu, F. Turkan, F. Şen, Antidiabetic and antiparasitic potentials: Inhibition effects of some natural antioxidant compounds on α-glycosidase, α-amylase and human glutathione S-transferase enzymes. Int. J. Biol. Macromol. 119, 741–746 (2018)
P. Taslimi, H.E. Aslan, Y. Demir, N. Oztaskin, A. Maraş, İ Gulçin, S. Beydemir, S. Goksu, Diarilmethanon, bromophenols and diarilmetan compounds: discovery of potent aldose reductase, α-amylase and α-glycosidase inhibitors as new therapeutic approach in diabetes and functional hyperglycemia. Int. J. Biol. Macromol. 119, 857–863 (2018)
A. Akıncıoğlu, S. Göksu, A. Naderi, H. Akıncıoğlu, N. Kılınç, İ Gülçin, Cholinesterases, carbonic anhydrase inhibitory properties and in silico studies of novel substituted benzylamines derived from dihydrochalcones. Comput. Biol. Chem. 94, 107565 (2021)
Schrödinger, Schrödinger Release 2020–3: Maestro, LLC, New York, NY, (2020)
G.M. Sastry, M. Adzhigirey, T. Day, R. Annabhimoju, W. Sherman, Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 27(3), 221–234 (2013)
W. Sherman, T. Day, M.P. Jacobson, R.A. Friesner, R. Farid, Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 49(2), 534–553 (2006)
M. Sandor, R. Kiss, G. Keserű, Virtual fragment docking by Glide: a validation study on 190 protein−fragment complexes. J. Chem. Inf. Model. 50(6), 1165–1172 (2010)
S. Genheden, U. Ryde, The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 10(5), 449–461 (2015)
N. Gök, A. Akıncıoğlu, E. Erümit Binici, H. Akıncıoğlu, N. Kılınç, S. Göksu, Synthesis of novel sulfonamides with anti‐Alzheimer and antioxidant capacities. Arch. Pharm. 354(7),e2000496 (2021)
F. Balestri, R. Moschini, U. Mura, M. Cappiello, A. Del Corso, In search of differential inhibitors of aldose reductase. Biomolecules 12(4), 485 (2022)
C.C. Zhao, X.H. Zhang, J. Chen, J.H. Shao, Z.Y. Zhao, Y.Y. Tang, Lignans with α-glucosidase, protein tyrosine phosphatase 1B, and aldose reductase inhibitory activities from the fruits of Viburnum cylindricum. Ind. Crops Prod. 178, 114601 (2022)
E. Bursal, A. Aras, Ö. Kılıç, P. Taslimi, A.C. Gören, İ Gulçin, Phytochemical content, antioxidant activity and enzyme inhibition effect of Salvia eriophora Boiss. & Kotschy against acetylcholinesterase, α-amylase, butyrylcholinesterase and α-glycosidase enzymes. J. Food Biochem. 43(3), e12776 (2019)
H. Shehryar, Kh.M. Khan, P. Taslimi, U. Salar, T. Taskin-Tok, D. Kisa, F. Saleem, M. Solangi, M.H. Ahmed, K. Rani, Evaluation of synthetic 2-aryl quinoxaline derivatives as α-amylase, α-glucosidase, acetylcholinesterase, and butyrylcholinesterase inhibitors. Int. J. Biol. Macromol. 211, 653–668 (2022)
N. Lolak, S. Akocak, C. Türkeş, P. Taslimi, M. Işık, Ş Beydemir, İ Gülçin, M. Durgun, Synthesis, characterization, inhibition effects, and molecular docking studies as acetylcholinesterase, α-glycosidase, and carbonic anhydrase inhibitors of novel benzenesulfonamides incorporating 1,3,5-triazine structural motifs. Bioorg. Chem. 100, 103897 (2020)
Y. Demir, P. Taslimi, Ü.M. Koçyiğit, M. Akkuş, M.S. Özaslan, H.E. Duran, Y. Budak, B. Tüzün, M.B. Gürdere, M. Ceylan, S. Taysi, İ Gülçin, Ş Beydemir, Determination of the inhibition profiles of pyrazolyl–thiazole derivatives against aldose reductase and α-glycosidase and molecular docking studies. Arch. Pharm. 353(12), 2000118 (2020)
İ Gulcin, O.V. Petrova, P. Taslimi, S.F. Malysheva, EYu. Schmidt, L.N. Sobenina, N.K. Gusarova, B.A. Trofimov, B. Tuzun, V.M. Farzaliyev, S. Alwasel, A.R. Sujayev, Synthesis, characterization, molecular docking, acetylcholinesterase and α-glycosidase inhibition profiles of nitrogen-based novel heterocyclic compounds. ChemistrySelect 7(19), e202200370 (2022)
P. Taslimi, A. Sujayev, M. Karaman, G. Maharramova, N. Sadeghian, S. Osmanova, S. Sardarova, N. Majdi, H.U. Ozel, İ Gulcin, N-Substituted pyrimidinethione and acetophenone derivatives as a new therapeutic approach in diabetes. Arch. Pharm. 353(7), 1–25 (2020)
A. Maharramov, M. Kurbanova, P. Taslimi, Y. Demir, A. Safarova, E. Huseyinov, A. Sujayev, S.H. Alwasel, İ Gulcin, Synthesis, characterization, crystal structure and bioactivities of novel enamine and pyrrole derivatives endowed with acetylcholinesterase, α-glycosidase and human carbonic anhydrase inhibition effects. Organic Commun. 14(2), 144–156 (2021)
Acknowledgements
This work was carried out with the financial support of the Science Development Fund under the President of the Republic of Azerbaijan—Grant NoEIF-ETL-2020-2(36)-16/11/4-m-11. Saleh H. Alwasel would like to extend his sincere appreciation to the Researchers Supporting Project (RSP-2024/59), King Saud University, Saudi Arabia, for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sujayev, A., Sadeghian, N., Taslimi, P. et al. Functionally substituted derivatives of novel thiourea and phenylthiourea as potent aldose reductase, α-amylase, and α-glycosidase inhibitors: in vitro and in silico studies. Macromol. Res. (2024). https://doi.org/10.1007/s13233-024-00247-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13233-024-00247-9