Abstract
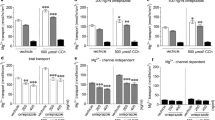
A commercial casein hydrolysate, which contains peptides RYLGY and AYFYPEL as active molecules, has shown antihypertensive effects after acute and long-term administration. This study examines transepithelial absorption of RYLGY and AYFYPEL and derived digestion fragments using Caco-2, HT29-MTX−6 and co-culture 75 % Caco-2/25 % HT29-MTX−6 as predictive models and RP-HPLC–MS as analytical tool. Peptides RYLGY and AYFYPEL were absorbed intact through cell monolayers, although RYLGY was partly hydrolyzed by intestinal peptidases. Co-culture 75 % Caco-2/25 % HT29-MTX−6 exhibited intermediate properties, with regard to transepithelial electrical resistance, peptide hydrolysis, and absorption of the studied peptides, between Caco-2 and HT29-MTX−6 pure cultures. Interestingly, mucus layer that covered completely HT29-MTX−6 and co-culture monolayers was not a barrier for the absorption of the studied peptides. The apparent permeability values for absorptive transport across Caco-2 monolayers for RYLGY (0.22 × 10−6 cm/s) and AYFYPEL (0.26 × 10−6 cm/s) were similar. These findings highlight that in vivo absorption of antihypertensive peptides RYLGY and AYFYPEL may occur partially as intact peptides.






Similar content being viewed by others
References
Avdeef A (2010) Leakiness and size exclusion of paracellular channels in cultured epithelial cell monolayers—interlaboratory comparison. Pharm Res 27:480–489
Behrens I, Stenberg P, Artursson P, Kissel T (2001) Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells. Pharm Res 18:1138–1145
Chua H-L, Seetharama J, Sim M-K, Go M-L (2004) Transport of angiotensin peptides across the Caco-2 monolayer. Peptides 25:1327–1338
Contreras MM (2010) Development of functional ingredients with antihypertensive peptides from milk proteins. PhD Dissertation, University Autonomous of Madrid
Contreras MM, Carrón R, Montero MJ, Ramos M, Recio I (2009) Novel casein-derived peptides with antihypertensive activity. Int Dairy J 19:566–573
Contreras MM, Gómez-Sala B, Martín-Álvarez PJ, Amigo L, Ramos M, Recio I (2010) Monitoring the large-scale production of the antihypertensive peptides RYLGY and AYFYPEL by HPLC-MS. Anal Bioanal Chem 397:2825–2832
Contreras MM, Sevilla MA, Monroy-Ruiz J, Amigo L, Gómez-Sala B, Molina E, Ramos M, Recio I (2011) Food-grade production of an antihypertensive casein hydrolysate and resistance of active peptides to drying and storage. Int Dairy J 21:470–476
Deferme S, Annaert P, Augustijns P (2008) In vitro screening models to assess intestinal drug absorption and metabolism. In: Erhardt C, Kim K-J (eds) Drug absorption studies—in situ, in vitro, and in silico models. Springer, New York, pp 182–215
Fleisher D (2000) Biological transport phenomena in the gastrointestinal tract: cellular mechanisms. In: Amidon GL, Lee PI, Topp EM (eds) Transport processes in pharmaceutical systems. Marcel Dekker Inc, New York, pp 147–184
Foltz M, Cerstiaens A, van Meensel A, Mols R, van der Pijl PC, Duchateau GSMJE, Augustijns P (2008) The angiotensin converting enzyme inhibitory tripeptides Ile-Pro-Pro and Val-Pro-Pro show increasing permeabilities with increasing physiological relevance of absorption models. Peptides 29:1312–1320
Foltz M, Meynen EE, Bianco V, van Platerink C, Koning TMMG, Kloek J (2007) Angiotensin converting enzyme inhibitory peptides from a lactotripeptide-enriched milk beverage are absorbed intact into the circulation. J Nutr 137:953–958
Granero GE, Longhi MR, Becker C, Junginger HE, Kopp S, Midha KK, Shah VP, Stavchansky S, Dressman JB, Barends DM (2008) Biowaiver monographs for immediate release solid oral dosage forms: acetazolamide. J Pharm Sci 97:3691–3699
Hennebicq Reig S, Kim I, Janin A, Grard G, Hémon B, Moreau O, Porchet N, Aubert JP, Degand P, Huet G (1996) Regulation of cathepsin D dependent on the phenotype of colon carcinoma cells. Int J Cancer 68:479–484
Hernández-Ledesma B, Contreras MM, Recio I (2011) Antihypertensive peptides: production, bioavailability and incorporation into foods. Adv Colloid Interf Sci 165:23–35
Hilgendorf C, Spahn-Langguth H, Regardh CG, Lipka E, Amidon GL, Langguth P (2000) Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: permeability via diffusion, inside- and outside-directed carrier-mediated transport. J Pharm Sci 89:63–75
Howell S, Kenny AJ, Turner AJ (1992) A survey of membrane peptidases in two human colonic cell lines, Caco-2 and HT-29. Biochem J 284:595–601
Iwan M, Jarmołowska B, Bielikowicz K, Kostyra E, Kostyra H, Kaczmarski M (2008) Transport of μ-opioid receptor agonists and antagonist peptides across Caco-2 monolayer. Peptides 29:1042–1047
Lai SK, Wang Y-Y, Hanes J (2009) Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev 61:158–171
Lei L, Sun H, Liu D, Liu L, Shimin L (2008) Transport of val-leu-pro-val-pro in human intestinal epithelial (Caco-2) cell monolayers. J Agric Food Chem 56:3582–3586
Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A (1990) Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res 50:6334–6343
Mahler GJ, Shuler ML, Glahn RP (2009) Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. J Nutr Biochem 20:494–502
Masuda O, Nakamura Y, Takano T (1996) Antihypertensive peptides are present in aorta after oral administration of sour milk containing these peptides in spontaneously hypertensive rats. J Nutr 126:3063–3068
Miguel M, Davalos A, Manso MA, de la Pena G, Lasuncion MA, López-Fandiño R (2008) Transepithelial transport across Caco-2 cell monolayers of antihypertensive egg-derived peptides. PepT1-mediated flux of Tyr-Pro-lle. Mol Nutr Food Res 52:1507–1513
Moreno FJ, Rubio LA, Olano A, Clemente A (2006) Uptake of 2S albumin allergens, Ber e 1 and Ses i 1, across human intestinal epithelial Caco-2 cell monolayers. J Agric Food Chem 54:8631–8639
Nollevaux G, Devillé C, El Moualij B, Zorzi W, Deloyer P, Schneider Y-J, Peulen O, Dandrifosse G (2006) Development of a serum-free co-culture of human intestinal epithelium cell-lines (Caco-2/HT29-5M21). BMC Cell Biol 7:20
Ohsawa K, Satsu H, Ohki K, Enjoh M, Takano T, Shimizu M (2008) Producibility and digestibility of antihypertensive β-casein tripeptides, Val-Pro-Pro and Ile-Pro-Pro, in the gastrointestinal tract: analyses using an in vitro model of mammalian gastrointestinal digestion. J Agric Food Chem 56:854–858
Pauletti GM, Gangwar S, Knipp GT, Nerurkar MM, Okumu FW, Tamura K, Siahaan TJ, Borchardt RT (1996) Structural requirements for intestinal absorption of peptide drugs. J Control Release 41:3–17
Pontier C, Pachot J, Botham R, Lenfant B, Arnaud P (2001) HT29-MTX and Caco-2/TC7 monolayers as predictive models for human intestinal absorption: role of the mucus layer. J Pharm Sci 90:1608–1619
Poquet L, Clifford MN, Williamson G (2008) Transport and metabolism of ferulic acid through the colonic epithelium. Drug Metab Dispos 36:190–197
Quirós A, Davalos A, Lasunción MA, Ramos M, Recio I (2008) Bioavailability of the antihypertensive peptide LHLPLP: transepithelial flux of HLPLP. Int Dairy J 18:279–286
Sánchez D, Kassan M, Contreras MM, Carrón R, Recio I, Montero M-J, Sevilla M-A (2011) Long-term intake of a milk casein hydrolysate attenuates the development of hypertension and involves cardiovascular benefits. Pharm Res 63:398–404
Satake M, Enjoh M, Nakamura Y, Takano T, Kawamura Y, Arai S, Shimizu M (2002) Transepithelial transport of the bioactive tripeptide, Val-Pro-Pro, in human intestinal Caco-2 cell monolayers. Biosci Biotechnol Biochem 66:378–384
Shimizu M, Tsunogai M, Arai S (1997) Transepithelial transport of oligopeptides in the human intestinal cell, Caco-2. Peptides 18:681–687
Sienkiewicz-Szlapka E, Jarmolowska B, Krawczuk S, Kostyra E, Kostyra H, Bielikowicz K (2009) Transport of bovine milk-derived opioid peptides across a Caco-2 monolayer. Int Dairy J 19:252–257
Sun H, Liu D, Li S, Qin Z (2009) Transepithelial transport characteristics of the antihypertensive peptide, Lys-Val-Leu-Pro-Val-Pro, in human intestinal Caco-2 cell monolayers. Biosci Biotechnol Biochem 73:293–298
Van der Pijl PC, Kies AK, Ten Have GAM, Duchateau GSMJE, Deutz NEP (2008) Pharmacokinetics of proline-rich tripeptides in the pig. Peptides 29:2196–2202
Van Platerink CJ, Janssen H-GM, Horsten R, Haverkamp J (2006) Quantification of ACE inhibiting peptides in human plasma using high performance liquid chromatography-mass spectrometry. J Chromatogr B 830:151–157
Vermeirssen V, van Camp J, Verstraete W (2004) Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br J Nutr 92:357–366
Walter E, Janich S, Roessler BJ, Hilfinger JM, Amidon GL (1996) HT29-MTX/Caco-2 cocultures as an in vitro model for the intestinal epithelium: in vitro-in vivo correlation with permeability data from rats and humans. J Pharm Sci 85:1070–1076
Zhu X-L, Watanabe K, Shiraishi K, Ueki T, Noda Y, Matsui T, Matsumoto K (2008) Identification of ACE-inhibitory peptides in salt-free soy sauce that are transportable across caco-2 cell monolayers. Peptides 29:338–344
Acknowledgments
This work has received financial support from the projects AGL2011-24643 and Consolider Ingenio 2010 FUN-C-Food CSD2007-00063 both from the Ministry of Economy and Competitivenes, and COST Action oc-2010-1-7087 (INFOGEST).
Conflict of Interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
del Mar Contreras, M., Sancho, A.I., Recio, I. et al. Absorption of Casein Antihypertensive Peptides through an In Vitro Model of Intestinal Epithelium. Food Dig. 3, 16–24 (2012). https://doi.org/10.1007/s13228-012-0020-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13228-012-0020-2