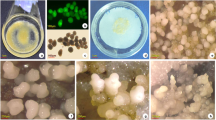
Abstract
Somatic embryogenesis is an important and wonderful biotechnological tool used to develop whole plant from a single or a group of somatic cells. The differentiated somatic cells become totipotent stem cells by drastic reprogramming of a wide range of cellular activities, leading to the acquisition of embryogenic competence. After acquiring competence, the cells pass through globular, heart, torpedo and cotyledonary stages of embryo; however, all advanced embryos do not convert into full plant, produce adventive embryos or callus instead, thus reverses the programming. This is a big limitation in propagation of many plants. Understanding and unraveling the proteins at this ‘embryo to plantlet’ transition stage will help to get more numbers of plants. Thus, our study was aimed at an identification of differentially abundant proteins between two important advanced stages, i.e. cotyledonary—(T1) and maturation stage (T2) of somatic embryos in Catharanthus roseus. A total of 2949 and 3030 proteins were identified in cotyledonary and maturation stage, respectively. Of these, 1129 proteins were common to both. Several proteins were found to be differentially accumulated in two different embryo stages in which over 60 proteins were most accumulated during somatic embryo maturation time. More chlorophyll accumulation was noted at this time under the influence of gibberellic acid (GA3). Proteins like Mg-protoporphyrin IX chelatase, chlorophyll a–b-binding protein, photosystem I iron-sulfur center, photosystem II Psb, photosystem II subunit P-1, P-II domain-containing protein, RuBisCO large chain, RuBisCO small chain, RuBisCO activase, RuBisCO large subunit-binding proteins were synthesized. Some of the identified proteins are linked to chlorophyll synthesis, carbohydrate metabolism and stress. The identified proteins are categorized into different groups on the basis of their cellular location, role and other metabolic processes. Biochemical attributes like protein, sugar, proline, antioxidant enzyme (APX, SOD and CAT) activities were high in T2 as compared to T1. The proteins like peroxidases, pathogenesis-related proteins, the late-embryogenesis abundant proteins, argonaute, germin and others have been discussed in C. roseus somatic embryo maturation process.







Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Altamura MM, Rovere FD, Fattorini L, D’Angeli S, Falasca G (2016) Recent advances on genetic and physiological bases of in vitro somatic embryo formation. In: Germana MA (ed) In vitro embryogenesis in higher plants. Springer, New York, pp 47–86
Amara I, Zaidi I, Masmoudi K, Ludevid MD, Pagès M, Goday A, Brini F (2014) Insights into late embryogenesis abundant (LEA) proteins in plants: from structure to the functions. Am J Pl Sci 5:3440–3455
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–253
Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu CM, van Lammeren AA, Miki BL, Custers JBM, van Lookeren Campagne MM (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749
Campos NA, Panis B, Carpentier SC (2017) Somatic embryogenesis in coffee: the evolution of biotechnology and the integration of omics technologies offer great opportunities. Front Plant Sci 8:1460
Cangahuala-Inocente GC, Steiner N, Santos M, Guerra MP (2004) Morphological analysis and histochemistry of Feijoa sellowiana somatic embryogenesis. Protoplasma 224:33–40
Chen J-B, Wang S-M, Jing R-L, Mao X-G (2009) Cloning the PvP5CS gene from common bean (Phaseolus vulgaris) and its expression patterns under abiotic stresses. J Plant Physiol 166:12–19
Cunha ACG, Ferreira MF (1996) Somatic embryogenesis, organogenesis and callus growth kinetics of flax. Plant Cell Tissue Org Cult 47(1):1–8
Curaba J, Moritz T, Blervaque R, Parcy F, Raz V, Herzog M, Vachon G (2014) AtGA3ox2, a key gene responsible for bioactive gibberellin biosynthesis, is regulated during embryogenesis by Leafy cotyledon2 and FUSCA3 in Arabidopsis. Plant Physiol 136:3660–3669
deMoura VE, Heringer AS, Barroso T, Ferreira AT, da Costa MN, Perales AJE, Santa-Catarina C, Silveira V (2014) Comparative proteomic analysis of somatic embryo maturation in Carica papaya L. Proteome Sci 12:37. https://doi.org/10.1186/1477-5956-12-37
Dey PM (1990) Methods in plant biochemistry. Carbohydrates, vol 2. Academic Press, London
Dhindsa RH, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot 32:93–101
Fehér A (2015) Somatic embryogenesis–stress-induced remodeling of plant cell fate. BiochimBiophysActa 1849(4):385–402
Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74:201–228
Fraga HP, Vieria LN, Heringer AS, Puttkammer CC, Silveira V, Guerra MP (2016) DNA methylation and proteome profiles of Araucaria angustfolia (Bertol) Kuntze embryogenic cultures as affected by plant growth regulators supplementation. Plant Cell Tissue Org Cult 125(2):353–374
Gaj MD, Zhang S, Harada JJ, Lemaux PG (2005) Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 222(6):977–988. https://doi.org/10.1007/s00425-005-0041-y
Gao J, Lan T (2015) Functional characterization of the late embryogenesis abundant (LEA) protein gene family from Pinus tabuliformis (Pinaceae) in Escherichia coli. Sci Rep 6:19467. https://doi.org/10.1038/srep19467
Gazzarrini S, Tsuchiya Y, Lumba S, Okamoto M, McCourt P (2004) The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Dev Cell 7:373–385
Ge X, Zhang C, Wang Q, Yang Z, Wang Y, Zhang X, Wu Z, Hou Y, Wu J, Li F (2015) iTRAQ protein profile differential analysis between somatic globular and cotyledonary embryos reveals stress, hormone, and respiration involved in increasing plantlet regeneration of Gossypium hirsutum L. J Proteome Res 14(1):268–278. https://doi.org/10.1021/pr500688g
Ge F, Hu H, Huang X, Zhang Y, Wang Y, Li Z, Zou C, Peng H, Li L, Gao S, Pan G, Shen Y (2017) Metabolomic and proteomic analysis of maize embryonic callus induced from immature embryo. Sci Rep 7(1):1004. https://doi.org/10.1038/s41598-017-01280-8
Guan Y, Li SG, Fan XF, Su ZH (2016) Application of somatic embryogenesis in woody plants. Front Plant Sci 7:938. https://doi.org/10.3389/fpls.2016.00938
Gulzar B, Mujib A, Rajam MV, Frukh A, Zafar N (2019) Identification of somatic embryogenesis (SE) related proteins through label-free shotgun proteomic method and cellular role in Catharanthusroseus (L.) G. Don. Plant Cell Tissue Org Cult 137(2):225–237
Gulzar B, Mujib A, Malik MQ, Syeed R, Mamgain J, Ejaz B (2020) Genes, proteins and other networks regulating somatic embryogenesis in plants. J Genet EngBiotechnol 18(1):1–15
Helleboid S, Hendriks T, Bauw G, InzeD VJ, Hilbert JL (2000) Three major somatic embryogenesis related proteins in Cichorium identified as PR proteins. J Exp Bot 51:1189–1200
Heringer AS, Barroso T, Macedo AF, Santa-Catarina C, Souza GHMF, Floh EIS, Souza-Filho GA, Silveira V (2015) Label-free quantitative proteomics of embryogenic and non-embryogenic callus during sugarcane somatic embryogenesis. PLoS ONE. https://doi.org/10.1371/journal.pone.0127803
Heringer AS, Santa-Catarina C, Silveira V (2018) Insights from proteomic studies into plant somatic embryogenesis. Proteomics. https://doi.org/10.1002/pmic.201700265
Isaacson T, Damasceno CM, Saravanan RS, He Y, Catala C, Saladie M, Rose JK (2006) Sample extraction techniques for enhanced proteomic analysis of plant tissues. Nat Protocol 2:769–774
Jin F, Hu L, Yuan D, Xu J, Gao W, He L (2014) Comparative transcriptome analysis between somatic embryos (SEs) and zygotic embryos in cotton: evidence for stress response functions in SE development. Plant Biotechnol J 12:161–173. https://doi.org/10.1111/pbi.12123
Jing D, Zhang J, Xia Y, Kong L, OuYang F, Zhang S, Zhang H, Wang J (2016) Proteomic analysis of stress-related proteins and metabolic pathways in Piceaasperata somatic embryos during partial desiccation. Plant Biotechnol J. https://doi.org/10.1111/pbi.12588
Junaid A, Mujib A, Fatima S, Sharma MP (2008) Cultural conditions affect somatic embryogenesis in Catharanthus roseus L. (G.) Don. Plant Biotechnol Rep 2:179–189
Kumaravel M, Uma S, Backiyarani S, Saraswathi MS (2019) Molecular analysis of somatic embryogenesis through proteomic approach and optimization of protocol in recalcitrant Musa spp. Physiol Plant 167:282–301
Kumaravel M, Uma S, Backiyarani S, Saraswathi MS (2020) Proteomic analysis of somatic embryo development in Musa spp. Cv. Grand Naine (AAA). Sci Rep. https://doi.org/10.1038/s41598-020-61005-2
Li Q, Zhang S, Wang J (2015) Transcriptomic and proteomic analyses of embryogenic tissues in Picea balfouriana treated with 6-benzylaminopurine. Physiol Plant 154(1):95–113. https://doi.org/10.1111/ppl.12276
Lu D, Wei W, Zhou W, Linda D, Yu XJ, L, (2017) Establishment of a somatic embryo regeneration system and expression analysis of somatic embryogenesis-related genes in Chinese chestnut (Castanea mollissima Blume). Plant Cell Tissue Org Cult 130(3):601–616
Ma Q, Ding Y, Chang J, Sun X, Zhang L, Wei Q, Cheng Y, Chen L, Xu J, Deng X (2014) Comprehensive insights on how 2,4-dichlorophenoxyacetic acid retards senescence in post-harvest citrus fruits using transcriptomic and proteomic approaches. J Exp Bot 65(1):61–74
Misic D, Siler B, Zivkovic NJ, Simonovic A, Maksimovic V, Budimir S, Janosevic D, Durickovic M, Nikolic M (2012) Contribution of inorganic cations and organic compounds to osmotic adjustment in root cultures of two Centaurium species differing in tolerance to salt stress. Plant Cell Tiss Org Cult 108:389–400
Misra RC, Sandeep KM, Kumar S, Ghosh S (2016) A thaumatin-like protein of Ocimum basilicum confers tolerance to fungal pathogen and abiotic stress in transgenic Arabidopsis. Sci Rep 6:25340. https://doi.org/10.1038/srep25340
Mujib A, Ali M, Isah T, Dipti T (2014) Somatic embryo mediated mass production of Catharanthus roseus in culture vessel (bioreactor)—a comparative study. Saudi J BiolSci 21(5):442–449
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Orłowska A, Igielski R, Łagowska K, Pczyska EK (2017) Identification of LEC1, L1L and Polycomb Repressive Complex2 genes and their expression during the induction phase of Medicago truncatulaGaertn. somatic embryogenesis. Plant Cell Tissue Org Cult 129:119–132. https://doi.org/10.1007/s11240-016-1161-8
Park CJ, Seo YS (2015) Heat shock proteins: a review of the molecular chaperones for plant immunity. Plant Path J 31(4):323–333
Patnaik D, Khurana P (2001) Germins and germin like proteins: an overview. Ind J ExpBiol 39:191–200
Radoeva T, Lokerse AS, Llavata-Peris CI, Wendrich JR, Xiang D, Liao CY, Vlaar L, Boekschoten M, Hooiveld G, Datla R et al (2019) A robust auxin response network controls embryo and suspensor development through a basic helix loop helix transcriptional module. Plant Cell 31:52–67
Rupps A, Raschke J, Rümmler M, Linke B, Zoglauer K (2016) Identification of putative homologs of Larix decidua to Babyboom (BBM), leafy cotyledon1 (LEC1), Wuschel-related Homeobox2 (WOX2) and somatic embryogenesis receptor-like kinase (SERK) during somatic embryogenesis. Planta 243:473–488
Schmidt ED, Guzzo F, Toonen MA, de Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124:2049–2062
Smulders MJM, Klerk GJ (2011) Epigenetics in plant tissue culture. Plant Growth Reg 63(2):137–146
Vale EM, Heringer AS, Barroso T, Ferreira ATDS, DaCosta MN, Perales JEA, Catarina CS, Silveira V (2014) Comparative proteomic analysis of somatic embryo maturation in Carica papaya L. Proteome Sci 12:1–17
Zhao J, Li H, Fu S, Chen B, Sun W, Zhang J (2015) AniTRAQ-based proteomics approach to clarify the molecular physiology of somatic embryo development in Prince Rupprecht’s larch (Larix principis-rupprechtiiMayr). PLoS ONE. https://doi.org/10.1371/journal.pone.0119987
Zhen Y, Li C, Chen J, Chen Q, Shi J (2015) Proteomics of embryogenic and non-embryogenic calli of a Liriodendron hybrid. ActaPhysiol Plant 37:211. https://doi.org/10.1007/s11738-015-1963-z
Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Acknowledgements
The first author is thankful to University Grant Commission (UGC) for receiving JRF. The authors are thankful to Department of Botany, Central Instrumentation facility, JamiaHamdard and University of Delhi for receiving necessary help.
Author information
Authors and Affiliations
Contributions
BG, NZ, JM, MM, RS designed, performed and analysed the experimental data. BG also wrote the draft of manuscript. AM and MVR edited the whole article.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in this article.
Ethical approval
This article did not involve any experiment or study with human participants or animals.
Rights and permissions
About this article
Cite this article
Gulzar, B., Mujib, A., Rajam, M.V. et al. Shotgun label-free proteomic and biochemical study of somatic embryos (cotyledonary and maturation stage) in Catharanthus roseus (L.) G. Don. 3 Biotech 11, 86 (2021). https://doi.org/10.1007/s13205-021-02649-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02649-3