Abstract
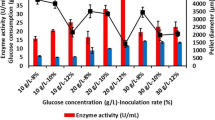
Polygalacturonases (PGs), an important industrial enzyme group classified under depolymerases, catalyze the hydrolytic cleavage of the polygalacturonic acid chain through the introduction of water across the oxygen bridge. In order to produce and increase the concentration of this enzyme group in fermentation processes, a new approach called microparticle cultivation, a promising and remarkable method, has been used. The aim of this study was to increase the PG activity of Aspergillus sojae using aluminum oxide (Al2O3) as microparticles in shake flask fermentation medium. Results indicated that the highest PG activity of 34.55 ± 0.5 U/ml was achieved with the addition of 20 g/L of Al2O3 while the lowest activity of 15.20 ± 0.2 U/mL was obtained in the presence of 0.1 g/L of Al2O3. In fermentation without microparticles as control, the activity was 15.64 ± 3.3 U/mL. Results showed that the maximum PG activity was 2.2-fold higher than control. Additionally, smaller pellets formed with the addition of Al2O3 where the lowest pellet diameter was 955.1 µm when 10 g/L of the microparticle was used. Also, it was noticed that biomass concentration gradually increased with increasing microparticle concentration in the fermentation media. Consequently, the PG activity was significantly increased in microparticle-enhanced shake flask fermentation. In fact, these promising preliminary data can be of significance to improve the enzyme activity in large-scale bioreactors.





Similar content being viewed by others
References
AlMatar M, Makky EA (2016) Cladosporium cladosporioides from the perspectives of medical and biotechnological approaches. 3 Biotech 6(1):4
Anand G, Yadav S, Yadav D (2016) Purification and characterization of polygalacturonase from Aspergillus fumigatus MTCC 2584 and elucidating its application in retting of Crotalaria juncea fiber. 3 Biotech 6(2):201
Antecka A, Bizukojc M, Ledakowicz S (2016a) Modern morphological engineering techniques for improving productivity of filamentous fungi in submerged cultures. World J Microbiol Biotechnol 32(12):193
Antecka A, Blatkiewicz M, Bizukojć M, Ledakowicz S (2016b) Morphology engineering of basidiomycetes for improved laccase biosynthesis. Biotech Lett 38(4):667–672
Botella C, Diaz A, De Ory I, Webb C, Blandino A (2007) Xylanase and pectinase production by Aspergillus awamori on grape pomace in solid state fermentation. Process Biochem 42(1):98–101
Coban HB, Demirci A (2016) Enhancement and modeling of microparticle-added Rhizopus oryzae lactic acid production. Bioprocess Biosyst Eng 39(2):323–330
Coban HB, Demirci A, Turhan I (2015a) Enhanced Aspergillus ficuum phytase production in fed-batch and continuous fermentations in the presence of talcum microparticles. Bioprocess Biosyst Eng 38(8):1431–1436
Coban HB, Demirci A, Turhan I (2015b) Microparticle-enhanced Aspergillus ficuum phytase production and evaluation of fungal morphology in submerged fermentation. Bioprocess Biosyst Eng 38(6):1075–1080
Demir H, Tari C (2016) Effect of physicochemical parameters on the polygalacturonase of an Aspergillus sojae mutant using wheat bran, an agro-industrial waste, via solid-state fermentation. J Sci Food Agri 96(10):3575–3582
Domingues FC, Queiroz JA, Cabral JMS, Fonseca LP (2000) The influence of culture conditions on mycelial structure and cellulase production by Trichoderma reesei Rut C-30. Enzyme Microbial Technol 26(5):394–401
Driouch H, Sommer B, Wittmann C (2010) Morphology engineering of Aspergillus niger for improved enzyme production. Biotechnol Bioeng 105(6):1058–1068
Driouch H, Roth A, Dersch P, Wittmann C (2011) Filamentous fungi in good shape: microparticles for tailor-made fungal morphology and enhanced enzyme production. Bioeng Bugs 2(2):100–104
Driouch H, Hänsch R, Wucherpfennig T, Krull R, Wittmann C (2012) Improved enzyme production by bio-pellets of Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol Bioeng 109(2):462–471
Etschmann M, Huth I, Walisko R, Schuster J, Krull R, Holtmann D, Wittmann C, Schrader J (2015) Improving 2-phenylethanol and 6-pentyl-α-pyrone production with fungi by microparticle-enhanced cultivation (MPEC). Yeast 32(1):145–157
Finkler ATJ, Biz A, Pitol LO, Medina BS, Luithardt H, de Lima Luz LF, Krieger N, Mitchell D (2017) Intermittent agitation contributes to uniformity across the bed during pectinase production by Aspergillus niger grown in solid-state fermentation in a pilot-scale packed-bed bioreactor. Biochem Eng J 121:1–12
Fratebianchi D, Crespo JM, Tari C, Cavalitto S (2017) Control of agitation rate and aeration for enhanced polygalacturonase production in submerged fermentation by Aspergillus sojae using agro-industrial wastes. J Chem Technol Biotechnol 92(2):305–310
Garg G, Singh A, Kaur A, Singh R, Kaur J, Mahajan R (2016) Microbial pectinases: an ecofriendly tool of nature for industries. 3 Biotech 6(1):1–13
Germec M, Yatmaz E, Karahalil E, Turhan İ (2017) Effect of different fermentation strategies on β-mannanase production in fed-batch bioreactor system. 3 Biotech 7(1):77
Gogus N, Tari C, Oncü S, Unluturk S, Tokatli F (2006) Relationship between morphology, rheology and polygalacturonase production by Aspergillus sojae ATCC 20235 in submerged cultures. Biochem Eng J 32(3):171–178
Gonciarz J, Bizukojc M (2014) Adding talc microparticles to Aspergillus terreus ATCC 20542 preculture decreases fungal pellet size and improves lovastatin production. Eng Life Sci 14(2):190–200
Hama S, Onodera K, Yoshida A, Noda H, Kondo A (2015) Improved production of phospholipase A 1 by recombinant Aspergillus oryzae through immobilization to control the fungal morphology under nutrient-limited conditions. Biochem Eng J 96:1–6
Heerd D, Diercks-Horn S, Fernandez-Lahore M (2014) Efficient polygalacturonase production from agricultural and agro-industrial residues by solid-state culture of Aspergillus sojae under optimized conditions. Springer Plus 3:742
Jørgensen TR (2007) Identification and toxigenic potential of the industrially important fungi, Aspergillus oryzae and Aspergillus sojae. J Food Prot 70(12):2916–2934
Kaup BA, Ehrich K, Pescheck M, Schrader J (2008) Microparticle-enhanced cultivation of filamentous microorganisms: increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnol Bioeng 99(3):491–498
Kumar S, Sharma H, Sarkar B (2011) Effect of substrate and fermentation conditions on pectinase and cellulase production by Aspergillus niger NCIM 548 in submerged (SmF) and solid state fermentation (SSF). Food Sci Biotechnol 20(5):1289–1298
Liu CT, Erh MH, Lin SP, Lo KY, Chen KI, Cheng KC (2016a) Enrichment of two isoflavone aglycones in black soymilk by Rhizopus oligosporus NTU 5 in a plastic composite support bioreactor. J Sci Food Agri 96(11):3779–3786
Liu J-M, Yu T-C, Lin S-P, Hsu R-J, Hsu K-D, Cheng K-C (2016b) Evaluation of kojic acid production in a repeated-batch PCS biofilm reactor. J Biotechnol 218:41–48
Meneghel L, Reis GP, Reginatto C, Malvessi E, da Silveira MM (2014) Assessment of pectinase production by Aspergillus oryzae in growth-limiting liquid medium under limited and non-limited oxygen supply. Process Biochem 49(11):1800–1807
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Nakkeeran E, Gowthaman MK, Umesh-Kumar S, Subramanian R (2012) Techno-economic analysis of processes for Aspergillus carbonarius polygalacturonase production. J Biosci Bioeng 113(5):634–640
Niu K, Hu Y, Mao J, Zou S, Zheng Y (2015) Effect of microparticles on echinocandin B production by Aspergillus nidulans. Sheng Wu Gong Cheng Xue Bao 31(7):1082–1088
Panda T, Naidu G, Sinha J (1999) Multiresponse analysis of microbiological parameters affecting the production of pectolytic enzymes by Aspergillus niger: a statistical view. Process Biochem 35(1):187–195
Sathya G, Naidu N, Panda T (1998) Application of response surface methodology to evaluate some aspects on stability of pectolytic enzymes from Aspergillus niger. Biochem Eng J 2(1):71–77
Sethi BK, Nanda PK, Sahoo S (2016) Enhanced production of pectinase by Aspergillus terreus NCFT 4269.10 using banana peels as substrate. 3 Biotech 6(1):1–15
Tari C, Gögus N, Tokatli F (2007) Optimization of biomass, pellet size and polygalacturonase production by Aspergillus sojae ATCC 20235 using response surface methodology. Enzyme Microbial Technol 40(5):1108–1116
Tokatli F, Tari C, Unluturk SM, Baysal NG (2009) Modeling of polygalacturonase enzyme activity and biomass production by Aspergillus sojae ATCC 20235. J Ind Microbiol Biotechnol 36(9):1139–1148
Tounsi H, Sassi AH, Romdhane ZB, Lajnef M, Dupuy J-W, Lapaillerie D, Lomenech A-M, Bonneu M, Gargouri A, Hadj-Taieb N (2016) Catalytic properties of a highly thermoactive polygalacturonase from the mesophilic fungus Penicillium occitanis and use in juice clarification. J Mol Catal B Enzym 127:56–66
Walisko R, Moench-Tegeder J, Blotenberg J, Wucherpfennig T, Krull R (2015) The taming of the shrew-controlling the morphology of filamentous eukaryotic and prokaryotic microorganisms. In: Krull R, Bley T (eds) Filaments in Bioprocesses. Springer, New York, pp 1–27
Wang L, Ridgway D, Gu T, Moo-Young M (2005) Bioprocessing strategies to improve heterologous protein production in filamentous fungal fermentations. Biotechnol Adv 23(2):115–129
Wolf-Márquez VE, Martínez-Trujillo MA, Osorio GA, Patiño F, Álvarez MS, Rodríguez A, Sanromán MÁ, Deive FJ (2017) Scaling-up and ionic liquid-based extraction of pectinases from Aspergillus flavipes cultures. Biores Technol 225:326–335
Yang J, Jiao R-H, Yao L-Y, Han W-B, Lu Y-H, Tan R-X (2016) Control of fungal morphology for improved production of a novel antimicrobial alkaloid by marine-derived fungus Curvularia sp. IFB-Z10 under submerged fermentation. Process Biochem 51(2):185–194
Yatmaz E, Karahalil E, Germec M, Ilgin M, Turhan İ (2016) Controlling filamentous fungi morphology with microparticles to enhanced β-mannanase production. Bioprocess Biosyst Eng 39(9):1391–1399
Acknowledgements
This study was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) Foundation Grant (#115 O 873). Authors also thank to the Akdeniz University Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors in this study mutually agree for submitting our manuscript to 3 Biotech and declare that they have no conflict of interest in the publication.
Rights and permissions
About this article
Cite this article
Karahalil, E., Demirel, F., Evcan, E. et al. Microparticle-enhanced polygalacturonase production by wild type Aspergillus sojae . 3 Biotech 7, 361 (2017). https://doi.org/10.1007/s13205-017-1004-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-1004-2