Abstract
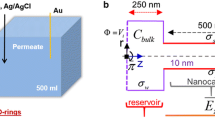
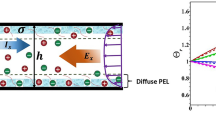
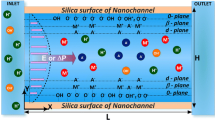
The ion concentration polarization (ICP) phenomenon occurs widely near nano-channel/membrane interfaces. Due to its extraordinary selective ion transport ability, ICP has been applied in many fields, such as desalination, molecular preconcentration and biomolecular separation. This paper is devoted to describing the transport mechanism of buffer ions at micro–nanochannel interfaces. Here, a multiphysics coupling model is proposed, where the boundary condition for the fixed surface voltage is introduced to describe the effect of nanochannel networks. The effectiveness of the proposed model and the calculation process is confirmed through comparative simulations. Comparing the simulations with experimental ICP results shows that the proposed model can effectively describe the nonlinear distribution of electric fields and a typical vortex pair from flow phenomena. An analytic scaling law for the propagating ion depletion zone (IDZ) is proposed, and a theoretical analysis and numerical results confirm its existence. For transient evolution, the IDZ spreads as \({\sqrt t }\) due to diffusion for t < 0.01 s and as t due to convection from 0.01 s < t < 0.1 s. Furthermore, detailed studies are performed to elucidate the ICP mechanism for desalination. The factors affecting desalination are investigated, including the buffer concentration, length and performance of the nanochannel network, height of the microchannel and the tangential electric field. Finally, the proposed research confirms that this device also has excellent potential as a micromixer pump. The rapid mixing of neutral particles can be realized using nonlinear electrokinetic flows with a mixing efficiency reaching 91%. The presented results provide some important guidance and physical insights into the design and optimization for this kind of chip and other related applications.







Similar content being viewed by others
Abbreviations
- C 0 :
-
Buffer concentration (M)
- C i :
-
Ion concentrations (M)
- C m :
-
Nanochannel surface concentration (M)
- C max :
-
Maximum in concentration mixture (M)
- D :
-
Diffusion coefficient (m2/s)
- E :
-
Electric field (V/m)
- e :
-
Elementary charge (C)
- F :
-
Faraday’s number (C/mol)
- g :
-
Gravitational acceleration (m/s2)
- H :
-
Characteristic length (m)
- J i :
-
Ion flux (mol/m2 s)
- L :
-
Microchannel length (m)
- L m :
-
Nanochannel network length (m)
- P :
-
Pressure (Pa)
- R :
-
Gas constant (J/(mol K))
- r :
-
Index of mixing
- T :
-
Absolute temperature (K)
- U :
-
Fluid velocity (m/s)
- V L :
-
Voltage at the left end of the channel (V)
- V sur :
-
Surface voltage (V)
- V R :
-
Voltage at the right end of the channel (V)
- V T :
-
Thermal voltage (V)
- Z i :
-
Ion valence
- \(\sigma_{ - }\) :
-
Negatively charged density (mC/m2)
- \(\beta\) :
-
Coefficient of expansion (kg/mol)
- \(\varepsilon_{0}\) :
-
Vacuum permittivity (F/m)
- \(\varepsilon_{\text{r}} (c)\) :
-
Solvent permittivity (F/m)
- \(\varepsilon_{\text{W}}\) :
-
Relative dielectric constant (1)
- \(\zeta (c)\) :
-
Zeta potential (V)
- \(\eta (c)\) :
-
Dynamic viscosity (Pa s)
- \(\rho (c)\) :
-
Concentration-dependent density (kg/m3)
- \(\rho_{0}\) :
-
Initial fluid density (kg/m3)
- \(\rho_{\text{e}}\) :
-
Space charged density (C/m3)
- \(\varPhi\) :
-
Electric potential (V)
- 1:
-
Cations
- 2:
-
Anions
- 3:
-
Particles
References
Chen Q, Jiang ZW, Yang ZH et al (2016) Differential-scheme based micromechanical framework for saturated concrete repaired by the electrochemical deposition method. Mater Struct 49(12):5183–5193
Chen Q, Jiang ZW, Yang ZH et al (2017) Differential-scheme based micromechanical framework for unsaturated concrete repaired by the electrochemical deposition method. Acta Mech 228:415–431
Chiu PH, Chang CC, Yang RJ (2013) Electrokinetic micromixing of charged and non-charged samples near nano–microchannel junction. Microfluid Nanofluid 14(5):839–844
Choi E, Kwon K, Lee SJ et al (2015) Non-equilibrium electrokinetic micromixer with 3d nanochannel networks. Lab Chip 15(8):1794–1798
Coelho D, Shapiro M, Thovert JF et al (1996) Electroosmotic phenomena in porous media. J Colloid Interface Sci 181(1):169–190
Demekhin EA, Shelistov VS, Polyanskikh SV (2011) Linear and nonlinear evolution and diffusion layer selection in electrokinetic instability. Phys Rev E 84(3):036318
Demekhin EA, Nikitin NV, Shelistov VS (2013) Direct numerical simulation of electrokinetic instability and transition to chaotic motion. Phys Fluids 25(12):122001
Deng DS, Wassim A, William AB, Bazant MZ et al (2015) Water purification by shock electrodialysis: deionization, filtration, separation, and disinfection. Desalination 357:77–83
Druzgalski CL, Andersen MB, Mani A (2013) Direct numerical simulation of electroconvective instability and hydrodynamic chaos near an ion-selective surface. Phys Fluids 25(11):8316–8322
Duan C, Majumdar A (2010) Anomalous ion transport in 2-nm hydrophilic nanochannels. Nat Nanotechnol 5:848–852
Dukhin SS (1991) Electrokinetic phenomena of the second kind and their applications. Adv Coll Interface Sci 35(91):173–196
Dydek EV, Zaltzman B, Rubinstein I et al (2011) Overlimiting current in a microchannel. Phys Rev Lett 107(11):118301
Hlushkou D, Knust KN, Crooks RM, Tallarek U (2016) Numerical simulation of electrochemical desalination. J Phys Condens Matter Inst Phys J 28(19):194001
Huang HN, Gao SB, Li X et al (2016) Microfluidic chips for effective desalination of seawater based on ionic concentration polarization. Chin J Anal Chem 44(12):1808–1813
Jia M, Kim T (2014) Multiphysics simulation of ion concentration polarization induced by a surface-patterned nanoporous membrane in single channel devices. Anal Chem 86(20):10365
Kalaĭdin EN, Polyanskikh SV, Demekhin EA (2010) Self-similar solutions in ion-exchange membranes and their stability. Dokl Phys 55(10):502–506
Karatay E, Druzgalski CL, Mani A (2015) Simulation of chaotic electrokinetic transport: performance of commercial software versus custom-built direct numerical simulation codes. J Colloid Interface Sci 446:67–76
Karatay E, Andersen MB, Wessling M, Mani A (2016) Coupling between buoyancy forces and electroconvective instability near ion-selective surfaces. Phys Rev Lett 116(19):194501
Kim SJ, Wang YC, Lee JH et al (2007) Concentration polarization and nonlinear electrokinetic flow near a nanofluidic channel. Phys Rev Lett 99(4):044501
Kim D, Raj A, Zhu L et al (2008) Non-equilibrium electrokinetic micro/nano fluidic mixer. Lab Chip 8:625–628
Kim SJ, Li LD, Han J (2009) Amplified electrokinetic response concentration polarization near nanofluidic channel. Langmuir 25(13):7759–7765
Kim SJ, Ko SH, Kang KH, Han J (2010) Direct seawater desalination by ion concentration polarization. Nat Nanotechnol 5(4):297–301
Lee J, Kim S, Kim C, Yoon J (2014) Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ Sci 7(11):3683–3689
Li Z, Liu W, Gong L et al (2017) Accurate multi-physics numerical analysis of particle preconcentration based on ion concentration polarization. Int J Appl Mech 9(08):1750107. https://doi.org/10.1142/S1758825117501071
Luo K, Wu J, Yi HL, Tan HP (2016) Lattice Boltzmann model for Coulomb-driven flows in dielectric liquids. Phys Rev E 93:1–11
Luo K, Wu J, Yi HL, Tan HP (2018) Three-dimensional finite amplitude electroconvection in dielectric liquids. Phys Fluids 30:023602
Mani A, Zangle TA, Santiago JG (2009) On the propagation of concentration polarization from microchannel–nanochannel interfaces. Part I: Analytical model and characteristic analysis. Langmuir 25(6):3898–3908
Marino S, Shapiro M, Adler PM (2001) Coupled transports in heterogeneous media. J Colloid Interface Sci 243(2):391–419
Mishchuk NA, Heldal T, Volden T et al (2009) Micropump based on electroosmosis of the second kind. Electrophoresis 30(20):3499–3506
Nikonenko VV, Kovalenko AV, Urtenov MK et al (2014) Desalination at overlimiting currents: state-of-the-art and perspectives. Desalination 342(5):85–106
Ouyang W, Ye X, Li Z et al (2018) Deciphering ion concentration polarization-based electrokinetic molecular concentration at the micro–nanofluidic interface: theoretical limits and scaling laws. Nanoscale. https://doi.org/10.1039/C8NR02170H
Peng R, Li D (2015) Effects of ionic concentration gradient on electroosmotic flow mixing in a microchannel. J Colloid Interface Sci 440:126–132
Pham VS, Li Z, Lim KM et al (2012) Direct numerical simulation of electroconvective instability and hysteretic current-voltage response of a permselective membrane. Phys Rev E 86(4 Pt 2):046310
Revil A, Pezard PA, Glover PWJ (1999a) Streaming potential in porous media: 1. theory of the zeta potential. J Geophys Res Atmos 104(B9):20021–20031
Revil A, Schwaeger H, Iii LMC et al (1999b) Streaming potential in porous media: 2. theory and application to geothermal systems. J Geophys Res Solid Earth 104(B9):20033–20048
Rubinstein II, Zaltzman B (2000) Electro-osmotically induced convection at a permselective membrane. Phys Rev E 62(2 Pt A):2238
Rubinstein I, Zaltzman B (2008) Electro-osmotic slip of the second kind and instability in concentration polarization at electrodialysis membranes. Math Models Methods Appl Sci 11(02):263–300
Rubinstein I, Zaltzman B (2015) Equilibrium electroconvective instability. Phys Rev Lett 114(11):114502
Shen M, Yang H, Sivagnanam V et al (2010) Microfluidic protein preconcentrator using a microchannel-integrated nafion strip: experiment and modeling. Anal Chem 82(24):9989
Shi PP, Liu W (2018) Length-dependent instability of shear electroconvective flow: from electroconvective instability to Rayleigh-Bénard instability. J Appl Phys 124(20):204304
Sung KB, Liao KP, Liu YL, Tian WC (2013) Development of a nanofluidic preconcentrator with precise sample positioning and multi-channel preconcentration. Microfluid Nanofluid 14(3–4):645–655
Wang YC, Stevens AL, Han J (2005) Million-fold preconcentration of proteins and peptides by nanofluidic filter. Anal Chem 77(14):4293–4299
Yeh LH, Zhang M, Qian S et al (2012) Ion concentration polarization in polyelectrolyte-modified nanopores. J Phys Chem C 116(15):8672–8677
Yossifon G, Chang HC (2008) Selection of nonequilibrium overlimiting currents: universal depletion layer formation dynamics and vortex instability. Phys Rev Lett 101(25):254501
Yu Q, Silber-Li Z (2011) Measurements of the ion-depletion zone evolution in a micro/nano-channel. Microfluid Nanofluid 11(5):623–631
Zangle TA, Mani A, Santiago JG (2009) On the propagation of concentration polarization from microchannel–nanochannel interfaces. Part II: Numerical and experimental study. Langmuir 25(6):3909–3916
Zangle TA, Mani A, Santiago JG (2010a) Effects of constant voltage on time evolution of propagating concentration polarization. Anal Chem 82(8):3114–3117
Zangle TA, Mani A, Santiago JG (2010b) Theory and experiments of concentration polarization and ion focusing at microchannel and nanochannel interfaces. Chem Soc Rev 39(3):1014
Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant Nos. 11972257, 11802225 and 11472193) and Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2019JQ-261). The authors gratefully acknowledge these supports. We also thank anonymous reviewers for their helpful comments on an earlier draft of this paper.
Author information
Authors and Affiliations
Corresponding authors
Appendix A: Numerical verification
Appendix A: Numerical verification
See Fig. 8.
Comparison of our presented results and the previous results. a Previous numerical results (Jia and Kim 2014). b Our presented numerical results
By neglecting the surface voltage boundary and no-slip boundary caused by the nanochannel network, the presented theoretical model can be directly compared to the simple model presented by Jia and Kim (2014) to prove the correctness of the numerical process. For this simplified case, an excellent agreement between the prediction from the present results and the previous simulation results is achieved. As a result, the present numerical calculation process has been validated.
Rights and permissions
About this article
Cite this article
Liu, W., Zhou, Y. & Shi, P. Electrokinetic ion transport at micro–nanochannel interfaces: applications for desalination and micromixing. Appl Nanosci 10, 751–766 (2020). https://doi.org/10.1007/s13204-019-01207-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-019-01207-x