Abstract
Wettability alteration is an important method to increase oil recovery from oil-wet carbonate reservoirs. Chemical agents are used as wettability modifiers in carbonate systems; however, the role of Nanoparticles in this field is still in its infancy and consequently has attracted the attention of many researchers in the last decade. In this work, the impact of SiO2 Nanoparticles on the wettability of a carbonate reservoir rock was experimentally studied. The impact of these Nanoparticles on the wettability of carbonate systems is still in its infancy. For this purpose, the effect of Nanofluid’s concentration on wettability and interfacial tension were investigated to determine the optimum concentration of Nanofluid for injection into core samples. The result suggests that a concentration of 4 g/L of Nanofluid could significantly alter the wettability of the rock from a strongly oil-wet to a strongly water-wet condition. Moreover, we studied the Nanofluids’ potential in enhanced oil recovery of oil-wet core plugs. The results show that a considerable amount of oil can be recovered right after start of water injection to the aged core plug with Nano fluid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowing that 50 % of the world’s known petroleum reserves are present in carbonate rocks, and that 90 % of these reservoirs are described as neutral to oil-wet, it is easy to understand the importance of research on these reservoirs (Roehl and Choquette 1985). The wetting properties of carbonate reservoirs are fundamental to the understanding of fluid flow through the porous media, and can affect the production characteristics greatly during water flooding. So, knowledge of the preferential wettability of reservoir rock is of upmost importance to petroleum engineers (Okasha et al. 2007). Due to this importance, many reviews of wettability and its effect on oil recovery have been conducted (Morrow 1990; Cuiec 1991; Vatanparast et al. 2011). After the primary production period, waterflooding is applied as a relatively cheap choice to improve oil recovery (Standnes and Austad 2000). Most carbonate reservoirs are preferentially oil wet, and therefore, the recovery of oil from these reservoirs by waterflooding techniques is relatively low (Anderson 1986; Buckley et al. 1998). The success to oil recovery improvement by waterflooding in carbonate systems is strongly on the wetting condition of the formation (Puntervold et al. 2007). Addition of chemical agents can modify rock wettability and therefore, increases the efficiency of waterflooding process. Numerous experimental works have been published discussing the role of chemical agents in wettability alteration of porous media (Austad and Standnes 2003; Strand et al. 2003; Standnes and Austad 2003; Hognesen et al. 2004; Mohan et al. 2011; Ravari et al. 2011; Tabrizy et al. 2011). However, the role of Nanoparticles in this field is still in its infancy and consequently has attracted the attention of many researchersin last decade due to their specific characteristics (Ju et al. 2002, 2006; Shen and Resasco 2009; Zhang et al. 2010; Suleimanov et al. 2011). Silicon dioxide (SiO2) Nanoparticles are very promising materials and can be used in the near future for enhanced oil recovery. These Nanoparticles have a great potential for increasing pore scale displacement efficiency (Ju and Fan 2009; Onyekonwu and Ologo 2010). Application of these Nanoparticles for enhancing oil recovery in water-wet sandstone systems is well documented in the literature (Ju et al. 2002, 2006; Ju and Fan 2009; Onyekonwu and Ologo 2010; Wang et al. 2010). However, from the view of the literature in this field, only few papers address the application of these Nanoparticles in wettability alteration of carbonate systems (Maghzi et al. 2011). In previous studies, the effect of Nanoparticles on oil–water interfacial tension and/or as an additive in waterflooding has not been investigated. The purpose of this paper is investigating the effect of SiO2 Nanoparticles in enhancing oil recovery from oil-wet carbonate system. The impact of Nanofluid’s different concentrations on alteration of wettability and change in oil–water interfacial tension is analyzed comprehensively to determine optimum concentration for injection into core samples. In addition, the impact of Nanoparticles on the amount of recovered oil is investigated using coreflood test.
Material and experiment procedure
Asmari reservoir formation samples were used as the rock sample obtained from one of the Iranian oil fields in the southwest arid land of Iran (i.e. Mansouri oilfield). Carbonate core plugs had a diameter of 3.84 cm and the lengths ranging from 8 to 8.50 cm, with porosity and average permeability equal to 16 % and 7 mD, respectively (Table 1). The composition of the brine used in all experiments was 5 wt% NaCl. The density and viscosity of the brine were 1.05 g/cm3 and 1.09 cP at room temperature, respectively. Crude oil was obtained from one of the oil fields in Khuzestan. The oil contains a little amount of asphaltene (1 wt%). Also, it has a viscosity of ~11.014 cP at 68 °F and API gravity of 33. Properties of SiO2 Nanoparticles are presented in Table 2. Different concentrations of Nanofluid (1–6 g/L) were used in this study prepared by sonication of Nanoparticles in brine for 80 min (600 W, 20 kHz). After Nanofluid preparation; the solutions were placed for 48 h in a closed transparent bottle away from degrading factors such as light and heat to ensure its homogeneity and stability.
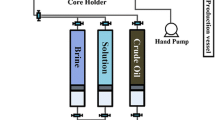
In order to examine Nanoparticles’ impact on wettability alteration, seven small carbonate plates (2 × 2 × 0.5 cm) were first cut from a cleaned sample using a trimming machine and polished to achieve a flat and relatively smooth surface. The plates were then aged for a week in the crude oil at 83 °C. After cooling, they were submerged in different concentrations of Nanofluid in room temperature for 2 h. Then, they were placed in a horizontal position surrounded by brine, and side images of oil drops on the carbonate plates were taken using a microscopic camera and contact angles were measured (Fig. 1). Oil–water and oil–Nanofluid interfacial tensions were measured in ambient pressure and room temperature (23 °C) by pendant drop method. To perform interfacial tension measurement, an oil droplet is allowed to hang from the tip of a capillary tube in a high pressure visual cell filled with brine and different concentrations of Nanofluid. Then, images of oil droplets were taken by a microscopic camera and interfacial tensions were measured (Danesh 1998). For coreflood tests, plugs were cleaned in a Dean-Stark apparatus for 4 days in contact with toluene vapor at ambient pressure. Then, they were evacuated for 60 min, saturated with brine. Afterward, the samples were placed into core holder and the brine was displaced by heavy oil until no brine is recovered. Core plugs were then aged in the oil at 80 °C for 20 days. After aging, heavy oil was displaced by six pore volume (PV) decahydronaphthalene (decalin) which in turn was flushed out by light oil and aged for 10 days at room temperature in order to restore the reservoir equilibrium condition. Then, coreflood experiments were performed under ambient pressure and room temperature with flow rate of 20 cc/h. The plugs were flooded with brine. Afterward, they were flooded with one PV of Nanofluid. In addition, the Nanofluid was aged for 24 h in the samples (Although we will show that wettability will be changed after 1 h but to ensure the full wettability alteration in the core samples, Nanofluid was rested for 24 h in the samples) and they were flooded again with brine.
Results and discussion
Contact angle
Wettability conditions were estimated before and after of surface modification with Nanoparticles, by measuring oil phase contact angle in presence of water. As mentioned before, oil phase contact angle of Nanofluids’ different concentrations (1–6 g/L) were examined to determine optimum value for concentration of Nanofluid for injection in core samples (Fig. 2). The maximum deviation between the contact angle data of each sample is found to be 5°. Therefore, the reported contact angle data in our experiments contain a maximum error of ±2.5°. Oil phase contact angle of the unmodified carbonate plate aged in the oil was 35°, implying a strong oil-wet condition. On the other hand, contact angles on the surface immersed plates with different concentrations of Nanoparticles increased to 130°, indicating an alteration in wettability to water-wet condition (Fig. 3). As can be clear seen, a stronger shift in wettability has been considerably improved by increasing Nanofluids’ concentration. A comparison between contact angle measurements for different concentrations of Nanofluids reveal that the concentration of 4 g/L has been more capable of changing the wettability to a water-wet condition. Wettability of a solid surface relates directly to the solid–fluids and fluid–fluid interactions. Although changes in fluid–fluid interactions might explain the wettability alteration, we speculate that this alteration is mainly due to solid fluid interaction. Nanoparticles adsorbing onto the rock surface affects its surface charge thereby altering wettability. The interfacial forces in a three-phase system relate to one another in a famous form known as Young’s law,
where θ is the oil phase contact angle in presence of brine and σ denotes interfacial tension. Superscripts sw, so, and wo represent the solid-water, solid–oil, and water–oil interfaces, respectively. As we will discuss later, presence of Nanoparticles at the oil–water interface provides an increase in oil–water interfacial tension. Therefore, according to Young’s law, Cosθ decreases linearly as the oil–water interfacial tension increased and consequently oil phase contact angle (θ) increases, implying transformation of system to water-wet condition. It should be noted that the impact of Nanoparticles on interfacial tension between brine/solid and oil/solid is not discussed in this paper. In addition, dynamics of wetting is investigated by oil phase contact angle measurement on carbonate plates being aged in concentration of 4 g/L of Nanofluid for various periods of time (Fig. 4). According to obtained results, a minimum of 60 min is required as the aging time for alteration of wettability from oil-wet to water-wet condition. To ensure about the results air/water contact angle on carbonate plate is also investigated. A similar conclusion can be drawn from measured air/water contact angle values (Fig. 5).
Interfacial tension
The change in interfacial tension between oil and water is very important, thus allowing the recovery of oil trapped in smaller pores and part of the residual oil remained in the pores after secondary flooding. For original wetting condition, reduction of interfacial tension will lead to a reduction of the capillary pressure within the pores and/or deformation of trapped oil. However, when wettability reversal to water-wet condition is the main mechanism for enhancing oil recovery, reduction of interfacial tension and consequently capillary pressure negatively influences the imbibition of water into small pores (Yu et al. 2008). In fact, after wettability alteration to water-wet condition, higher capillary pressure will lead to a stronger imbibition of water into small pores and consequently higher oil recovery.
As Fig. 6 illustrates, oil–water interfacial tension ascends with an increase in concentration of Nanofluid. Employing Nanofluid increases oil–water interfacial tension from 26.5 mN/m to around 38.4 mN/m. According to interfacial tension measurement results for different concentrations of Nanofluid, a concentration of 4 g/L shows the significant share in increasing of interfacial tension in proportion to its concentration. It was considered as the optimum concentration and employed in coreflood experiments. Increase in interfacial tension demonstrates Nanoparticles’ potential to increase the capillary pressure and consequently oil recovery after wettability reversal to water-wet condition. The mechanistic reason for this increase in oil–water interfacial tension is unknown to us, though it is certainly related to the interactions between fluids at the interface. However, adsorption of Nanoparticle at the interface can be a possible explanation for the observed phenomena.
Oil recovery
In the present study two sets of flooding scheme have been conducted after waterflooding. In the first set, enhanced recovery over Nanofluid flooding has been studied using approximately 1 PV of Nanofluid. In other set, water has been injected in the core sample after aging of Nanofluid for 24 h. As Fig. 7 illustrates, waterflooding recovery for first and second core sample is 42 and 47 %, respectively. It should be noted that oil recovery before breakthrough is 27 % for the first sample and 26 % for second one. Production of considerable amount of oil after breakthrough indicates oil-wet characteristic of samples (Anderson 1986).
Oil recovery increases by 9 and 12 %, respectively in first and second core sample during injection of one pore volume of Nanofluid and it increases by 16 and 17 %, respectively when Nanofluid is aged for 24 h in the core samples. Therefore, total oil recovery hits to more than 67 and 76 %, respectively in both samples which demonstrates the considered Nanofluid’s potential to improve oil recovery. A comparison between recovery results after injection of Nanofluid in both samples reveals that Nanoparticles can produce significant amount of oil after primary and secondary recovery process.
Mechanism
The residual oil after waterflood which is target of Nanofluid injection process is believed filling the smaller pores, as a continuous film over rock surfaces and as a larger packet of oil trapped and surrounded by water (Anderson 1987). The injection of Nanofluid into core samples can improve oil recovery by alteration of wettability from oil-wet to water-wet condition
The combined effects of wettability and interfacial tension cause the wetting fluid to be simultaneously imbibed into a capillary tube. Interfacial tension controlled the curvature of the oil–water interface and the value of capillary pressure. In addition, wettability governed the direction of capillary pressure. Therefore, it is ideal to increase capillary pressure value and reverse its direction by wettability alteration in carbonate reservoirs. According to results of interfacial tension and contact angle, when Nanoparticles are introduced to the porous media, some of them may adsorb on the rock surface, reverse wettability to water-wet condition and others may adsorb at the oil–water interface and increase interfacial tension and consequently capillary pressure. Capillary pressure which is negative in oil-wet condition increases with an increase in interfacial tension. However, it can be changed from negative to positive value after altering the wetting state of the rock surface which then leads to stronger imbibition of water in small pores. In fact, during waterflooding, small pores remain upswept due to oil-wet wettability and high capillary pressure. In the case of small pores, capillary pressure hinders entrance of wetting phase into pores. However, when Nanoparticles are adsorbed on surface of pore throat, alteration of rock wettability to water-wet condition reverse the direction of capillary force and cause strong imbibition of wetting phase into small pores and depleted them (Yu et al. 2008).
Conclusions
In this work, we present results of SiO2 Nanofluids’ impact on enhanced oil recovery during coreflood experiments on oil-wet carbonate samples. Based on the obtained results the following conclusions can be drawn:
-
1.
SiO2 Nanoparticles are wettability modifiers for carbonate systems, and they can change the wettability of carbonate rocks from strongly oil-wet to strongly water-wet condition.
-
2.
Wettability change by adsorption of Nanoparticles on the rock surface is a fast process, requiring a period of at least 1 h.
-
3.
Use of these Nanoparticles in flooding tests revealed the strong capability of these new agents for oil recovery from carbonate hydrocarbon reservoirs. The mechanism of oil recovery by selected Nanofluid during Nanofluid flooding and after aging of Nanofluid attributed to wettability alteration.
References
Anderson WG (1986) Wettability literature survey-part 2: wettability measurement. J Pet Technol 38:1246–1262
Anderson WG (1987) Wettability literature survey, part 6. The effects of wettability on waterflooding. J Pet Technol 39:1605–1622
Austad T, Standnes DC (2003) Spontaneous imbibition of water into oil-wet carbonates. J Pet Sci Eng 39:363–376
Buckley JS, Liu Y, Monsterleet S (1998) Mechanisms of wetting alteration by crude oils. SPE J 3:54–61
Cuiec LE (1991) Evaluation of reservoir wettability and its effect on oil recovery. In: Morrow NR (ed) Interfacial phenomena in oil recovery. Marcel Dekker, New York, pp 375–391
Danesh A (1998) PVT and phase behaviour of petroleum reservoir fluids. Elsevier, The Netherlands
Hognesen E, Standnes DC, Austad T (2004) Scaling spontaneous imbibition of aqueous surfactant solution into preferential oil-wet carbonates. Energy Fuels 18:1665–1675
Ju B, Fan T (2009) Experimental study and mathematical model of nanoparticle transport in porous media. Powder Technol 192:195–202
Ju B, shugao D, Zhian L, Tiangao Z, Xiantao S, Xiaofeng Q, (2002) A study of wettability and permeability change caused by adsorption of nanometer structured polysilicon on the surface of porous media, Peresented at SPE Asia Pacific Oil and Gas Conference and Exhibition, Melbourne, Australia, SPE Paper No. 77938
Ju B, Fan T, Ma M (2006) Enhanced oil recovery by flooding with hydrophilic nanoparticles. Chin Part 4:41–46
Maghzi A, Mohebbi A, Kharrat R, Ghazanfari MH (2011) Pore-scale monitoring of wettability alteration by silica nanoparticles during polymer flooding to heavy oil in a five-spot glass micromodel. Trans Porous Med 87:653–664
Mohan K, Gupta R, Mohanty KK (2011) Wettability altering secondary oil recovery in carbonate rocks. Energy Fuels 25:3966–3973
Morrow NR (1990) Wettability and its effect on oil recovery. J Pet Tech 42:1476–1484
Okasha TM, Funk JJ, Al-Rashidi HN (2007). Fifty years of wettability measurements in the arab-D carbonate reservoir, Presented at SPE Middle East Oil and Gas Show and Conference, Bahrain, SPE Paper No. 105114-MS
Onyekonwu M, Ologo N (2010) Investigating the use of nanoparticles in enhancing oil recovery, Presented at Annual SPE International Conference and Exhibition. Tinapa, Calabar, Nigeria, SPE Paper No. 140744
Puntervold T, Strand S, Austad T (2007) Water flooding of carbonate reservoirs: effects of a model base and natural crude oil bases on chalk wettability. Energy Fuels 21:1606–1616
Ravari RR, Strand S, Austad T (2011) Combined surfactant-enhanced gravity drainage (SEGD) of oil and the wettability alteration in carbonates: the effect of rock permeability and interfacial tension (IFT). Energy Fuels 25:2083–2088
Roehl PO, Choquette PW (1985) Carbonate petroleum reservoirs. Springer, New York
Shen M, Resasco DE (2009) Emulsions stabilized by carbon nanotube-silica nanohybrids. Langmuir 25:10843–10851
Standnes DC, Austad T (2000) Wettability alteration in chalk: 2. Mechanism for wettability alteration from oil-wet to water-wet using surfactants. J Pet Sci Eng 28:123–143
Standnes DC, Austad T (2003) Wettability alteration in carbonates: interaction between cationic surfactant and carboxylates as a key factor in wettability alteration from oil-wet to water-wet conditions. Colloids Surf 216:243–259
Strand S, Standnes DC, Austad T (2003) spontaneous imbibition of aqueous surfactant solutions into neutral to oil-wet carbonate cores: effects of brine salinity and composition. Energy Fuels 17:1133–1144
Suleimanov BA, Ismailov FS, Veliyev EF (2011) Nanofluid for enhanced oil recovery. J Pet Sci Eng 78:431–437
Tabrizy VA, Hamouda AA, Denoyel R (2011) Influence of magnesium and sulfate ions on wettability alteration of calcite, quartz, and kaolinite: surface energy analysis. Energy Fuels 25:1667–1680
Vatanparast A, Alizadeh H, Bahramian A, Bazder H (2011) Wettability alteration of low-permeable carbonate reservoir rocks in presence of mixed ionic surfactants. J Pet Sci Tech 29:1873–1884
Wang K, Liang Sh, Wang C (2010) Research of improving water injection effect by using active SiO2 nano-powder in the low-permeability oilfield. Adv Mat Res 92:207–212
Yu L, Evje S, Kleppe H, Karstad T, Fjelde I, Skjaeveland SM, (2008) Analysis of the wettability alteration process during seawater imbibition into preferentially oil-wet chalk cores, Presented at SPE/DOE Improved Oil Recovery Symposium. Tulsa, USA, SPE Paper No. 113304
Zhang T, Davidson D, Bryant SL, Huh C (2010) Nanoparticle-stabilized emulsions for applications in enhanced oil recovery, Presented at SPE Improved Oil Recovery Symposium. Tulsa, USA, SPE Paper No. 129885-MS
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Roustaei, A., Bagherzadeh, H. Experimental investigation of SiO2 nanoparticles on enhanced oil recovery of carbonate reservoirs. J Petrol Explor Prod Technol 5, 27–33 (2015). https://doi.org/10.1007/s13202-014-0120-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-014-0120-3