Abstract
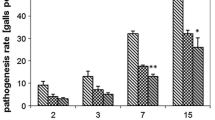
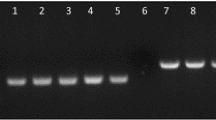
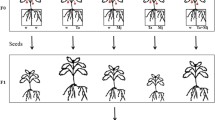
Pochonia chlamydosporia (Pc123) is a fungal parasite of nematode eggs which can colonize endophytically barley and tomato roots. In this paper we use culturing as well as quantitative PCR (qPCR) methods and a stable GFP transformant (Pc123gfp) to analyze the endophytic behavior of the fungus in tomato roots. We found no differences between virulence/root colonization of Pc123 and Pc123gfp on root-knot nematode Meloidogyne javanica eggs and tomato seedlings respectively. Confocal microscopy of Pc123gfp infecting M. javanica eggs revealed details of the process such as penetration hyphae in the egg shell or appressoria and associated post infection hyphae previously unseen. Pc123gfp colonization of tomato roots was low close to the root cap, but increased with the distance to form a patchy hyphal network. Pc123gfp colonized epidermal and cortex tomato root cells and induced plant defenses (papillae). qPCR unlike culturing revealed reduction in fungus root colonization (total and endophytic) with plant development. Pc123gfp was found by qPCR less rhizosphere competent than Pc123. Endophytic colonization by Pc123gfp promoted growth of both roots and shoots of tomato plants vs. uninoculated (control) plants. Tomato roots endophytically colonized by Pc123gfp and inoculated with M. javanica juveniles developed galls and egg masses which were colonized by the fungus. Our results suggest that endophytic colonization of tomato roots by P. chlamydosporia may be relevant for promoting plant growth and perhaps affect managing of root-knot nematode infestations.




Similar content being viewed by others
References
Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EGJ, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC (2008) Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat biotechnol 26(8):909–915
Atkins SD, Peteira B, Clark IM, Kerry BR, Hirsch PR (2009) Use of real-time quantitative PCR to investigate root and gall colonisation by co-inoculated isolates of the nematophagous fungus Pochonia chlamydosporia. Ann Appl Biol 155(1):143–152
Bailey DJ, Biran GL, Kerry BR, Gilligan CA (2008) Pathozone dynamics of Meloidogyne incognita in the rhizosphere of tomato plants in the presence and absence of the nematophagous fungus, Pochonia chlamydosporia. Plant Pathol 57(2):354–362
Barelli L, Padilla-Guerrero IE, Bidochka MJ (2011) Differential expression of insect and plant specific adhesin genes, Mad1 and Mad2, in Metarhizium robertsii. Fungal Biol 115(11):1174–1185
Bird AF, Bird J (1991) The structure of nematodes. The egg. Academic, San Diego, pp 7–43
Bordallo JJ, Lopez-Llorca LV, Jansson HB, Salinas J, Persmark L, Asensio L (2002) Colonization of plant roots by egg-parasitic and nematode-trapping fungi. New Phytol 154(2):491–499
Bourne JM, Kerry BR (1999) Effect of the host plant on the efficacy of Verticillium chlamydosporium as a biological control agent of root-knot nematodes at different nematode densities and fungal application rates. Soil Biol and Biochem 31:75–84
Bourne JM, Kerry BR, De Leig FAAM (1996) The importance of the host plant on the interaction between root-knot nematodes Meloidogyne spp. and the nematophagous fungus, Verticillium chlamydosporium goddard. Biocontrol Sci Tech 6(4):539–548
Byrd DW Jr, Kirkpatrick T, Barker KR (1983) An improved technique for clearing and staining plant tissues for detection of nematodes. J Nematol 15(1):142
Ciancio A, Loffredo A, Paradies F, Turturo C, Sialer MF (2005) Detection of Meloidogyne incognita and Pochonia chlamydosporia by fluorogenic molecular probes*. EPPO Bull 35(1):157–164
Gayoso C, Ilárduya OM, Pomar F, Merino de Caceres F (2007) Assessment of real-time PCR as a method for determining the presence of Verticillium dahliae in different Solanaceae cultivars. Eur J Plant Pathol 118(3):199–209
Hellwig V, Mayer-Bartschmid A, Müller H, Greif G, Kleymann G, Zitzmann W, Tichy HV, Stadler M (2003) Pochonins A-F, New Antiviral and Antiparasitic Resorcylic Acid Lactones from Pochonia chlamydosporia var. catenulata. J Nat Prod 66:829–837
Hidalgo-Díaz L, Bourne JM, Kerry BK, Rodríguez MG (2000) Nematophagous Verticillium spp. in soils infested with Meloidogyne spp. in Cuba: Isolation and screening. Int J Pest Manage 46(4):277–284
Hierro N, Esteve-Zarzoso B, González A, Mas A, Guillamón JM (2006) Real-time quantitative PCR (QPCR) and reverse transcription-QPCR for detection and enumeration of total yeasts in wine. Appl Environ Microl 72(11):7148–7145
Inglis PW, Aragao FJL, Frazao H, Magalhaes BP, Valadares-Inglis MC (2000) Biolistic co-transformation of Metarhizium anisopliae var. acridum strain CG423 with green fluorescent protein and resistance to glufosinate ammonium. FEMS Microbiol Lett 191:249–254
Kerry B, Hidalgo-Diaz L (2004). Application of Pochonia chlamydosporia in the integrated control of root-knot nematodes on organically grown vegetable crops in Cuba. Bulletin OILB/SROP 27:123–126
Kerry BR, Bourne JM (2002). A manual for Research on Verticillium chlamdosporium, as Potential Biological Control Agent for Root-Knot Nematodes. Ed WPRS/SROP
Kerry BR, Crump DH (1977) Observations on fungal parasites of females and eggs of the cereal cyst-nematode, Heterodera avenae, and other cyst nematodes. Nematologica 23:193–201
Khambay B, Bourne JM, Cameron S, Kerry B, Zaki M (2000) A nematicidal metabolite from Verticillium chlamydosporium. Pest Manag Sci 56:1098–1099
Kurtti TJ, Keyhani NO (2008) Intracellular infection of tick cell lines by the entomopathogenic fungus Metarhizium anisopliae. Microbiology 154(6):1700–1709
Lee S, Kim SH, Breuil C (2002) The use of the green fluorescent protein as a biomarker for sapstain fungi. Forest Pathol 32(3):153–161
Lopez-Llorca LV, Boag B (1993) Biological properties of a red pigment produced by the nematophagous fungus Verticillium suchlasporium. Nematol medit 21:143–149
Lopez-Llorca LV, Claugher D (1990) Appressoria of the nematophagous fungus Verticillium suchlasporium. Micron Microscoc Acta 21(3):125–130
Lopez-Llorca LV, Duncan JM (1986) New media for the estimation of fungal infection in eggs of the cereal cyst nematode. Nematologica 32:486–490
Lopez-Llorca LV, Duncan GH (1987) A study of fungal endoparasitism of the cereal cyst nematode Heterodera avenae by scanning electron microscopy. Can J Microbiol 34:613–619
Lopez-Llorca LV, Robertson WM (1992) Ultrastructure of infection of cyst nematode eggs by the nematophagous fungus Verticillium Suchlasporium. Nematologica 39(4):65–74
Lopez-Llorca LV, Bordallo JJ, Salinas J, Monfort E, Lopez-Serna ML (2002) Use of Light and scanning electron microscopy to examine colonisation of barley rhizosfere by the nematophagous fungus Verticillium chlamydosporium. Micron 33:61–67
Lopez-Llorca LV, Jansson HB, Maciá-Vicente JG, Salinas J (2006) Nematophagous fungi as root endophytes. In: Microbial root endophytes, Springer–Verlag, pp 191–206
Lopez-Llorca LV, Gomez-Vidal S, Monfort E, Larriba E, Casado-Vela J, Elortza F, Jansson HB, Salinas J, Martín-Nieto J (2010) Expression of serine proteases in egg-parasitic nematophagous fungi during barley root colonization. Fungal Genet Biol 47(4):342–351
Maciá-Vicente JG, Jansson HB, Talbot NJ, Lopez-Llorca LV (2009a) Real-time PCR quantification and live-cell imaging of endophytic colonization of barley (Hordeum vulgare) roots by Fusarium equiseti and Pochonia chlamydosporia. New Phytol 182(1):213–228
Maciá-Vicente JG, Rosso LC, Ciancio A, Jansson HB, Lopez-Llorca LV (2009b) Colonisation of barley roots by endophytic Fusarium equiseti and Pochonia chlamydosporia: Effects on plant growth and disease. Ann Appl Biol 155(3):391–401
Manzanilla-López RH, Esteves I, Powers SJ, Kerry BR (2011) Effects of crop plants on abundance of Pochonia chlamydosporia and other fungal parasites of root-knot and potato cyst nematodes. Ann Appl Biol 159(1):118–129
McClure MA, Kruk TH, Misaghi I (1973) A method for obtaining quantities of clean Meloidogyne eggs. J Nematol 5(3):230
Monfort E, Lopez-Llorca LV, Jansson HB, Salinas J, Park JO, Sivasithamparam K (2005) Colonisation of seminal roots of wheat and barley by egg-parasitic nematophagous fungi and their effects on Gaeumannomyces graminis var. tritici and development of root-rot. Soil Biol Biochem 37(7):1229–1235
Niu XM, Wang YL, Chu YS, Xue HX, Li N, Wei LX, Mo MH, Zhang KQ (2010) Nematodetoxic aurovertin-type metabolites from a rook-knot nematode parasitic fungus Pochonia chlamydosporia. J Agric Food Chem 58:828–834
Nordbring-Hertz B, Jansson HB, Tunlid A (2006) Encyclopedia of life sciences. pp 1–11
Olivares-Bernabeu CM, Lopez-Llorca LV (2002) Fungal egg-parasites of plant-parasitic nematodes from Spanish soils. Rev Iberoam Micol 19(2):104–110
Persmark L, Jansson HB (1997) Nematophagous fungi in the rhizosphere of agricultural crops. FEMS Microbiol Ecol 22(4):303–312
Segers R, Butt MT, Kerry BR, Beckett A, Peberdy JF (1996) The role of the proteinase VCP1 produced by the nematophagous Verticillium chlamydosporium in the infection process of nematode eggs. Mycol Res 100:421–428
Siddiqi ZA, Akhtar MS (2008) Synergistic effects of antagonistic fungi and a plant growth promoting rhizobacterium, an arbuscular mycorrhizal fungus, or composted cow manure on populations of Meloydogyne incognita and growth of tomato. Biocontrol Sci Techn 18(3):207–290
Siddiqui IA, Ehteshamul-Haque S (2000) Effect of Verticillium chlamydosporium and Pseudomonas aeruginosa in the control of Meloidogyne javanica in tomato. Nematol Medit 28:193–196
Siddiqui IA, Shaukat SS (2003) Combination of Pseudomonas aeruginosa and Pochonia chlamydosporia for Control of Root-Infecting Fungi in Tomato. J Phytopathology 151:215–222
Sikora RA, Pocasangre L, Felde A, Niere B, Vu TT, Dababat AA (2008) Mutualistic endophytic fungi and in-planta suppressiveness to plant parasitic nematodes. Biol Control 46:15–23
Sorribas FJ, Ornat C, Galeano M, Verdejo-Lucas S (2003) Evaluation of a Native and Introduced Isolate of Pochonia chlamydosporia against Meloidogyne javanica. Biocontrol Sci Techn 13(8):707–714
Stirling GR (2011) Biological control of plant-parasitic nematodes: an ecological perspective, a review of progress and opportunities for further research. Biological Control of Plant-Parasitic Nematodes. pp 1–38
Tobin JD, Haydock PPJ, Hare MC, Woods SR, Crump DH (2008) Effect of the fungus Pochonia chlamydosporia and fosthiazate on the multiplication rate of potato cyst nematodes (Globodera pallida and G. rostochiensis) in potato crops grown under UK field conditions. Biol Control 46(2):194–201
Tzortzakakis EA, Petsas SE (2003) Investigation of alternatives to methyl bromide for management of Meloidogyne javanica on greenhouse grown tomato. Pest Manag Sci 59:1311–1320
Van Damme V, Hoedekie A, Viaene N (2005) Long-term efficacy of Pochonia chlamydosporia for management of Meloidogyne javanica in glasshouse crops. Nematology 7:741–745
Verdejo-Lucas S, Sorribas FJ, Galeno M (2003) Evaluating Pochonia chlamydosporia in a double-cropping system of lettuce and tomato in plastic houses infested with Meloidogyne javanica. Plant Pathol 52:521–528
Wesemael WML, Viaene N, Moens M (2010) Root-knot nematodes (Meloidogyne spp.) in Europe. Nematology 13(1):3–16
Wiedenmann J, Oswald F, Nienhaus GU (2009) Fluorescent proteins for live cell imaging: Opportunities, limitations, and challenges. IUBMB Life 61(11):1029–1042
Zhang L, Yang J, Niu Q, Zhao X, Ye F, Liang L, Zhang KQ (2008) Investigation on the infection mechanism of the fungus Clonostachys rosea against nematodes using the green fluorescent protein. Appl Microbiol Biot 78(6):983–990
Zijlstra C, Donkers-Venne D, Fargette M (2000) Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplifed region (SCAR) based PCR assays. Nematology 2(8):847–853
Acknowledgements
This research was funded by the Spanish Ministry of Science and Innovation Grants AGL 2008-00716/AGR and AGL 2011-29297. We thank Mr. E. Larriba (University of Alicante, Spain) for designing qPCR primers for VcP1 gene and Dr. F. J. Sorribas (Universitat Politècnica de Catalunya) for his help in the establishment of the nematode population. We also thank Professor Hans-Börje Jansson (University of Alicante) for critical comments on the manuscript.
In Memoriam
This paper is dedicated to the memory of the late Prof. Brian Kerry (Rothamsted Research, UK). He made key discoveries in the field of nematophagous fungi finding that soil suppression to plant parasitic nematodes was due to fungal parasites of nematode eggs. His work and scientific thought inspired the research of biologists all over the world including us. Many thanks Brian!
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escudero, N., Lopez-Llorca, L.V. Effects on plant growth and root-knot nematode infection of an endophytic GFP transformant of the nematophagous fungus Pochonia chlamydosporia . Symbiosis 57, 33–42 (2012). https://doi.org/10.1007/s13199-012-0173-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-012-0173-3