Abstract
Purpose
Integrin αv is a key regulator in the pathophysiology of hepatic fibrosis. In this study, we evaluated the potential utility of an integrin αvβ3 positron emission tomography (PET) radiotracer, 18F-labeled cyclic arginine-glycine-aspartic acid penta-peptide ([18F]F-FPP-RGD2), for detecting hepatic integrin αv and function in nonalcoholic steatohepatitis (NASH) model rats using integrin αv siRNA.
Methods
NASH model rats were produced by feeding a choline-deficient, low-methionine, high-fat diet for 8 weeks. PET/computerized tomography imaging and quantification of integrin αv protein, serum aspartate aminotransferase, and alanine aminotransferase were performed 1 week after single intravenous injection of integrin αv siRNA.
Results
Integrin αv siRNA (0.1 and 0.5 mg/kg) dose-dependently decreased hepatic integrin αv protein concentrations in control and NASH model rats. The hepatic mean standard uptake value of [18F]F-FPP-RGD2 was decreased dose-dependently by integrin αv siRNA. The mean standard uptake value was positively correlated with integrin αv protein levels in control and NASH model rats. Serum aspartate aminotransferase and alanine aminotransferase concentrations were also decreased by siRNA injection and correlated with liver integrin αv protein expression levels in NASH model rats.
Conclusion
This study suggests that [18F]F-FPP-RGD2 PET imaging is a promising radiotracer for monitoring hepatic integrin αv protein levels and hepatic function in NASH pathology.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Schuppan D, Surabattula R, Wang XY. Determinants of fibrosis progression and regression in NASH. J Hepatol. 2018;68:238–50.
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411.
Maile LA, Busby WH, Nichols TC, Bellinger DA, Merricks EP, Rowland M, et al. A monoclonal antibody against alphaVbeta3 integrin inhibits development of atherosclerotic lesions in diabetic pigs. Sci Transl Med. 2010;2.
Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9:804–20.
Gerber EE, Gallo EM, Fontana SC, Davis EC, Wigley FM, Huso DL, et al. Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature. 2013;503:126–30.
Turaga RC, Satyanarayana G, Sharma M, Yang JJ, Wang S, Liu C, et al. Targeting integrin αvβ3 by a rationally designed protein for chronic liver disease treatment. Commun Biol. 2021;4:1–10.
Patsenker E, Popov Y, Stickel F, Schneider V, Ledermann M, Sägesser H, et al. Pharmacological inhibition of integrin alphavbeta3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis. Hepatology. 2009;50:1501–11.
Li F, Song Z, Li Q, Wu J, Wang J, Xie C, et al. Molecular imaging of hepatic stellate cell activity by visualization of hepatic integrin αvβ3 expression with SPECT in rat. Hepatology. 2011;54:1020–30.
Zhou X, Murphy FR, Gehdu N, Zhang J, Iredale JP, Benyon RC. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem. 2004;279:23996–4006.
Turaga RC, Yin L, Yang JJ, Lee H, Ivanov I, Yan C, et al. Rational design of a protein that binds integrin αvβ3 outside the ligand binding site. Nat Commun. 2016;7:1–11.
Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology. 2008;135:660–70.
Rokugawa T, Konishi H, Ito M, Iimori H, Nagai R, Shimosegawa E, et al. Evaluation of hepatic integrin αvβ3 expression in non-alcoholic steatohepatitis (NASH) model mouse by 18F-FPP-RGD2 PET. EJNMMI Res. 2018;8:40.
Hiroyama S, Rokugawa T, Ito M, Iimori H, Morita I, Maeda H, et al. Quantitative evaluation of hepatic integrin αvβ3 expression by positron emission tomography imaging using 18F-FPP-RGD2 in rats with non-alcoholic steatohepatitis. EJNMMI Res. 2020;10.
Haskali MB, Roselt PD, Karas JA, Noonan W, Wichmann CW, Katsifis A, et al. One-step radiosynthesis of 4-nitrophenyl 2-[(18) F]fluoropropionate ([(18) F]NFP); improved preparation of radiolabeled peptides for PET imaging. J Labelled Comp Radiopharm. 2013;56:726–30.
Bergeron M, Cadorette J, Tétrault MA, Beaudoin JF, Leroux JD, Fontaine R, et al. Imaging performance of LabPET APD-based digital PET scanners for pre-clinical research. Phys Med Biol. 2014;59:661–78.
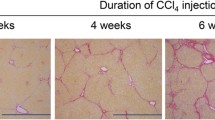
Matsumoto M, Hada N, Sakamaki Y, Uno A, Shiga T, Tanaka C, et al. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int J Exp Pathol. 2013;94:93–103.
Van Herck MA, Vonghia L, Francque SM. Animal models of nonalcoholic fatty liver disease-a starter’s guide. Nutrients. 2017;9.
Zhang C, Liu H, Cui Y, Li X, Zhang Z, Zhang Y, et al. Molecular magnetic resonance imaging of activated hepatic stellate cells with ultrasmall superparamagnetic iron oxide targeting integrin αvβ3 for staging liver fibrosis in rat model. Int J Nanomedicine. 2016;11:1097–108.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Kossen K, Schaefer C, Lim S, Michener M, Griggs D, Ruminski P, et al. IDL-2965: a selective, highly potent, clinical-stage integrin antagonist for treatment of non-alcoholic steatohepatitis. J Hepatol. 2020;73:S450.
Kapp TG, Rechenmacher F, Neubauer S, Maltsev OV, Cavalcanti-Adam EA, Zarka R, et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci Rep. 2017;7:1–13.
Acknowledgements
We acknowledge members of the Imaging Group at Shionogi & Co., Ltd. for their constructive discussion. We also thank Ellen Knapp, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript, with funding from Shionogi & Co., Ltd.
Funding
This study was funded by Shionogi & Co., Ltd.
Author information
Authors and Affiliations
Contributions
SH performed the PET experiments and data analysis and drafted the manuscript. MI performed the PET experiments. HI synthesized the PET probe. IM performed the protein and AST/ALT analysis. JN performed the design and synthesization of LNP formulation. KM and ES were the supervisors of this study. KA coordinated and designed the study and drafted the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Shuichi Hiroyama, Keiko Matsunaga, Miwa Ito, Hitoshi Iimori, Ippei Morita, Jun Nakamura, Eku Shimosegawa, and Kohji Abe declare that they have no competing interests.
Ethics Approval
The experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Shionogi & Co. Ltd. and Osaka University Graduate School of Medicine.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hiroyama, S., Matsunaga, K., Ito, M. et al. Evaluation of an Integrin αvβ3 Radiotracer, [18F]F-FPP-RGD2, for Monitoring Pharmacological Effects of Integrin αv siRNA in the NASH Liver. Nucl Med Mol Imaging 57, 172–179 (2023). https://doi.org/10.1007/s13139-023-00791-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-023-00791-9