Abstract
Purpose
2-Deoxy-2-[18F] fluoro-d-glucose positron emission tomography (18F-FDG-PET) is a less-invasive and widely used diagnostic tool for detection of malignant tumors. However, prolonged retention of 18F-FDG in the body increases radiation exposure. This study evaluated the effect of oral administration of milk and ursodeoxycholic acid (UDCA) in terms of reducing radiation exposure by 18F-FDG.
Methods
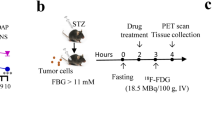
18F-FDG radioactivity was measured using a digital γ counter in the whole body and in various organs of rats after oral administration of milk and milk plus UDCA (milk + UDCA). Western blotting was performed to measure the expression levels of G6Pase, HK 2, CREB, FoxO1, and PGC-1α in the brain, liver, small intestine, and large intestine to assess the mechanism underlying the reduction in radiation exposure from 18F-FDG by oral administration of milk and UDCA.
Results
We found a significant reduction in 18F-FDG radioactivity in the whole body and in the brain, liver, and small and large intestines. Expression of G6Pase was significantly increased in the above-mentioned organs in the milk and milk + UDCA groups. Expression of HK 2 was significantly decreased in the brain and small intestine in the milk and milk + UDCA groups. CREB, FoxO1, and PGC-1α expression levels in the brain, liver, and small intestine were increased in the milk and milk + UDCA groups. However, expression of PGC-1α in the large intestine in the milk and milk + UDCA groups was significantly decreased compared with that in the control group.
Conclusion
The present study demonstrated that administration of milk and UDCA increased G6Pase expression levels and 18F-FDG release from the tissue. These results suggest milk and UDCA could be used to reduce radiation exposure from 18F-FDG after image acquisition. The mechanisms underpinning this phenomenon should be explored in a human study.







Similar content being viewed by others
References
Kostakoglu L, Agress H Jr, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics. 2003;23:315–40 quiz 533.
Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallires E, et al. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000;6:3837–44.
Zincirkeser S, Sahin E, Halac M, Sager S. Standardized uptake values of normal organs on 18F-fluorodeoxyglucose positron emission tomography and computed tomography imaging. J Int Med Res. 2007;35:231–6.
Conti PS, Lilien DL, Hawley K, Keppler J, Grafton ST, Bading JR. PET and [18F]-FDG in oncology: a clinical update. Nucl Med Biol. 1996;23:717–35.
Huang B, Law MW, Khong PL. Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology. 2009;251:166–74.
Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10.
Chen X, Iqbal N, Boden G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest. 1999;103:365–72.
Massillon D, Barzilai N, Hawkins M, Prus-Wertheimer D, Rossetti L. Induction of hepatic glucose-6-phosphatase gene expression by lipid infusion. Diabetes. 1997;46:153–7.
Wahli W, Braissant O, Desvergne B. Peroxisome proliferator activated receptors: transcriptional regulators of adipogenesis, lipid metabolism and more. Chem Biol. 1995;2:261–6.
Jeong HJ, Kim CG. Pretreatment with ursodeoxycholic acid (UDCA) as a novel pharmacological intervention in hepatobiliary scintigraphy. Yonsei Med J. 2005;46:394–8.
Alam MS, Teshima S, Ishikawa M, Koshio S, Ohtao H. The role of ursodeoxycholic acid on growth performance and digestive enzyme activities of tilapia Oreochromis niloticus and kuruma prawn Marsupenaeus japonicus. Asian Fisheries Science Journal. 2001;14:441–51.
Bagalkot TR, Jin HM, Prabhu VV, Muna S.S, Cui Y, Yadav BK et al. Chronic social defeat stress increases dopamine D2 receptor dimerization in the prefrontal cortex of adult mice. Neuroscience. 2015;311:444–452.
MacGibbon AHK, Taylor MW. Composition and structure of bovine milk lipids. In: Fox PF, McSweeney PLH, editors. Advanced dairy chemistry. New York: Springer; 2006. p. 1–42.
Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic trapping as a principle of radiopharmaceutical design: some factors responsible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med. 1978;19:1154–61.
Scrutton M, Utter M. The regulation of glycolysis and gluconeogenesis in animal tissues. A Rev Biochem. 1968;37:249–302.
Anchors JM, Karnovsky ML. Purification of cerebral glucose-6-phosphatase. An enzyme involved in sleep. J Biol Chem. 1975;250:6408–16.
Colilla W, Jorgenson RA, Nordlie RC. Mammalian carbamyl phosphate : glucose phosphotransferase and glucose-6-phosphate phosphohydrolase: extended tissue distribution. Biochim Biophys Acta. 1975;377:117–25.
Karnovsky ML. Possible involvement of cerebral glucose-6-phosphatase in 2-deoxy-D-glucose phosphorylation. Relationship of 2-deoxy-D-glucose phosphorylation to local cerebral energy utilization. Neurosci Res Program Bull. 1976;14:505–8.
Stephens HR, Sandborn EB. Cytochemical localization of glucose-6-phosphatase activity in the central nervous system of the rat. Brain Res. 1976;113:127–46.
Hahn P, Wei-Ning H. Gluconeogenesis from lactate in the small intestinal mucosa of suckling rats. Pediatr Res. 1986;20:1321–3.
Ockerman PA. Glucose-6-phosphatase in human jejunal mucosa properties demonstrating the specific character of the enzyme activity. Biochim Biophys Acta. 1965;105:22–33.
Mithieux G. New knowledge regarding glucose-6 phosphatase gene and protein and their roles in the regulation of glucose metabolism. Eur J Endocrinol. 1997;136:137–45.
Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–83.
Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–8.
Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–35.
Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10:530–4.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0139).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Hwan-Jeong Jeong, Tarique Rajasaheb Bagalkot, Hyeon Soo Kim, Yeon-Hee Han, Minjoo Kim, Seok Tae Lim, and Myung-Hee Sohn declare that they have no conflict of interest.
Ethical Approval
All protocols involving animals were conducted in compliance with the policy and procedure of the Institutional Animal Care and Use Committee of Jeonbuk National University (CBNU 2015-097) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeong, HJ., Bagalkot, T.R., Kim, H.S. et al. Radioactivity Reduction of 2-Deoxy-2-[18F] Fluoro-D-Glucose by Milk and Ursodeoxycholic Acid in Preclinical Study. Nucl Med Mol Imaging 54, 105–113 (2020). https://doi.org/10.1007/s13139-020-00634-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-020-00634-x