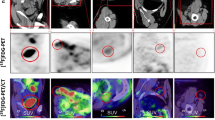
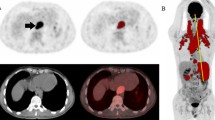
Abstract
Purpose
Based on the International Harmonization Project (IHP) criteria, positron emission tomography (PET) response assessment of residual nodal masses in patients with lymphoma after completion of therapy is performed visually using mediastinal blood pool as the reference. The primary objective of this study was to define the optimal reference for PET response assessment. Secondary aim was to assess if morphological criteria on computed tomography (CT) may improve performance of PET.
Methods
This institutional review board approved retrospective study included 137 patients, with Hodgkin’s (n = 43) or non-Hodgkin’s lymphoma (n = 94) assessed for residual masses (n = 180) after completion of therapy with pathology and clinical and imaging surveillance data (mean, 19 months) as the standard of reference. Two readers independently assessed response by IHP and Deauville criteria. The addition of morphological parameters on CT was assessed in relation to therapy response.
Results
Based on the standard of reference, 36 patients (26.3 %) had residual lymphoma. For IHP and Deauville criteria, sensitivity, specificity and accuracy were 97.2 %, 97.2 % (p = 1); 79.2 %, 92.1 % (p < 0.001); and 83.9 %, 93.4 % (p = 0.001), respectively. Of the morphological parameters assessed, only change in size over course of therapy was significant (p < 0.003) and improved specificity for IHP-based interpretation to 90.4 % (p = 0.008).
Conclusions
Using liver as the visual reference to determine PET positivity for lymphoma patients being assessed for residual masses at the end of therapy improves specificity, yet maintains the high sensitivity of PET in identifying residual disease. The addition of change in size after therapy improves specificity of PET when using IHP-based but not Deauville-based interpretation.

Similar content being viewed by others
References
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244–53.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8.
Juweid M, Wiseman GA, Vose JM, Ritchie JM, Menda Y, Wooldridge JE, et al. Response assessment of aggressive non-Hodgkin’s lymphoma by integrated International Workshop criteria (IWC) and 18F-fluorodeoxyglucose positron emission tomography (PET). J Clin Oncol. 2005;23:4652–61.
Bangerter M, Moog F, Buchmann I, Kotzerke J, Griesshammer M, Hafner M, et al. Whole-body 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for accurate staging of Hodgkin’s disease. Ann Oncol. 1998;9:1117–22.
Spaepen K, Stroobants S, Dupont P, Van Steenweghen S, Thomas J, Vandenberghe P, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin’s lymphoma: Is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol. 2001;19:414–9.
Spaepen K, Stroobants S, Dupont P, Vandenberghe P, Maertens J, Bormans G, et al. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003;102:53–9.
Spaepen K, Stroobants S, Dupont P, Vandenberghe P, Thomas J, de Groot T, et al. Early restaging positron emission tomography with 18F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2002;13:1356–63.
Jerusalem G, Beguin Y, Fassotte MF, Najjar F, Paulus P, Rigo P, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose compared to standard procedures for staging patients with Hodgkin’s disease. Haematologica. 2001;86:266–73.
Jerusalem G, Beguin Y, Fassotte MF, Najjar F, Paulus P, Rigo P, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin’s disease and non-Hodgkin’s lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood. 1999;94:429–33.
Jerusalem G, Beguin Y, Najjar F, Hustinx R, Fassotte MF, Rigo P, et al. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) for the staging of low-grade non-Hodgkin’s lymphoma (NHL). Ann Oncol. 2001;12:825–30.
Jerusalem G, Warland V, Najjar F, Paulus P, Fassotte MF, Fillet G, et al. Whole-body 18F-FDG PET for the evaluation of patients with Hodgkin’s disease and non-Hodgkin's lymphoma. Nucl Med Commun. 1999;20:13–20.
Zinzani PL, Magagnoli M, Chierichetti F, Zompatori M, Garraffa G, Bendandi M, et al. The role of positron emission tomography (PET) in the management of lymphoma patients. Ann Oncol. 1999;10:1141–3.
Naumann R, Vaic A, Beuthien-Baumann B, Bredow J, Kropp J, Kittner T, et al. Prognostic value of positron emission tomography in the evaluation of post-treatment residual mass in patients with Hodgkin’s disease and non-Hodgkin’s lymphoma. Br J Haematol. 2001;115:793–800.
Munker R, Glass J, Griffeth LK, Sattar T, Zamani R, Heldmann M, et al. Contribution of PET imaging to the initial staging and prognosis of patients with Hodgkin’s disease. Ann Oncol. 2004;15:1699–704.
Mikhael NG, Hutchings M, Fields PA, Nunan T, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514–23.
Juweid M, Cheson BD. Positron emission tomography (PET) in post-therapy assessment of cancer. N Engl J Med. 2006;354:496–507.
Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–81.
Hutchings M, Loft A, Hansen M, Pedersen LM, Berthelsen AK, Keiding S, et al. Positron emission tomography with or without computed tomography in the primary staging of Hodgkin’s lymphoma. Haematologica. 2006;91:482–9.
Olsen K, Sohi J, Abraham T, Juweid M. Initial validation of standardized qualitative (visual) criteria for FDG-PET assessment of residual masses following lymphoma therapy. Radiological Society of North America 92nd Scientific Assembly and Annual Meeting Program, 2006, pp 323 (abstract 55:E23-02).
Brepoels L, Stroobants S, De Wever W, Spaepen K, Vandenberghe P, Thomas J, et al. Hodgkin lymphoma: response assessment by revised International Workshop Criteria. Leuk Lymphoma. 2007;48:1539–47.
Brepoels L, Stroobants S, De Wever W, Spaepen K, Vandenberghe P, Thomas J, et al. Aggressive and indolent non-Hodgkin’s lymphoma: response assessment by integrated international workshop criteria. Leuk Lymphoma. 2007;48:1522–30.
Gallamini A, Rigacci L, Merli F, Nassi L, Bosi A, Capodanno I, et al. Predictive value of positron emission tomography performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin’s disease. Haematologica. 2006;91:475–81.
Meignan M, Barrington S, Itti E, Gallamini A, Haioun C, Polliack A. Report on the 4th International Workshop on Positron Emission Tomography in Lymphoma held in Menton, France, 3–5 October 2012. Leuk Lymphoma. 2014;55(1):31–7.
Itti E, Meignan M, Berriolo-Riedinger A, Biggi A, Cashen AF, Véra P, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging. 2013;40:1312–20.
Dupuis J, Berriolo-Riedinger A, Julian A, Brice P, Tychyj-Pinel C, Tilly H, et al. Impact of [(18)F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: a prospective study from the Groupe d’Etudes des Lymphomes de l’Adulte and GOELAMS. J Clin Oncol. 2012;30:4317–22.
Manohar K, Mittal BR, Raja S, Bhattacharya A, Malhotra P, Varma S. Comparison of various criteria in interpreting end of therapy F-18 labeled fluorodeoxyglucose positron emission tomography/computed tomography in patients with aggressive non-Hodgkin lymphoma. Leuk Lymphoma. 2013;54:714–9.
Kajáry K, Molnár Z, Györke T, Szakáll Jr S, Molnár P, Lengyel Z. Comparison of the International Harmonization Project, London and Gallamini criteria in the interpretation of 18F-FDG PET/CT examinations after first-line treatment in Hodgkin’s lymphoma. Nucl Med Commun. 2014;35:169–75.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Barrington SF, Qian W, Somer EJ, Franceschetto A, Begni B, Brun E, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:1824–33.
Kinahan PE, Fletcher JW. PET/CT standardized Uptake Values (SUVs) in Clinical Practice and Assessing Response to Therapy. Semin Ultrasound CT MR. 2010;31:496–505.
Doot RK, Scheuermann JS, Christian PE, Karp JS, Kinahan PE. Instrumentation factors affecting variance and bias of quantifying tracer uptake with PET/CT. Med Phys. 2010;37:6035–46.
Kobe C, Kuhnert G, Kahraman D, Haverkamp H, Eich HT, Franke M, et al. Assessment of tumor size reduction improves outcome prediction of positron emission tomography/computed tomography after chemotherapy in advanced-stage Hodgkin lymphoma. J Clin Oncol. 2014;32:1776–81.
Han HS, Escalon MP, Hsiao B, Serafini A, Lossos IS. High incidence of false-positive PET scans in patients with aggressive non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Ann Oncol. 2009;20:309–18.
Shacter E, Williams JA, Hinson RM, Sentürker S, Lee YJ. Oxidative stress interferes with cancer chemotherapy: inhibition of lymphoma cell apoptosis and phagocytosis. Blood. 2000;96:307–13.
Acknowledgments
This manuscript has not been published before, is not under consideration for publication anywhere else and has been approved by all co-authors.
Conflict of Interest
All authors (Ur Metser, Ravi Mohan, Vaughan Beckley, Hadas Moshonov, David Hodgson, and Grainne Murphy) declare that they have no conflict of interest.
Ethical Statement
This retrospective study was approved by the institution’s Research Ethics Board (approval no. 13-6496-CE) and written informed consent from patients was waived. The study was performed in accordance with the ethical standards of the institution. The institutional research ethics board operates in compliance with the Tri-Council Policy Statement and the ICH guidelines for the Good Clinical Practice E6(R1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Metser, U., Mohan, R., Beckley, V. et al. FDG PET/CT Response Assessment Criteria for Patients with Hodgkin’s and Non-Hodgkin’s Lymphoma at End of Therapy: A Multiparametric Approach. Nucl Med Mol Imaging 50, 46–53 (2016). https://doi.org/10.1007/s13139-015-0368-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-015-0368-7