Abstract
Purpose
Preclinical studies in rats showed that two of 99mTc(CO)3(ASMA) isomers (rac- and L-ASMA) had pharmacokinetic properties equivalent to that of 131I-OIH, the radiopharmaceutical standard for the measurement of effective renal plasma flow. The aim of this study was to evaluate the pharmacokinetics of 99mTc(CO)3(ASMA) isomers in healthy human subjects.
Methods
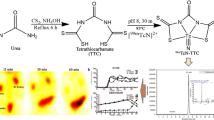
Three ASMA ligands (rac-, L- and D-ASMA) were labeled with 99mTc(CO)3 using an IsoLink kit (Covidien), and each formed 99mTc(CO)3(ASMA) tracer was co-injected with 131I-OIH into healthy human subjects followed by sequential imaging, plasma clearance measurements and timed urine collection. Plasma protein binding, red cell uptake and percent injected dose in the urine were determined. Urine from each group of volunteers was analyzed for metabolites by HPLC.
Results
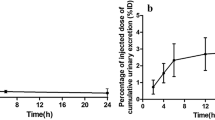
Image quality was excellent with all three agents. Each 99mTc(CO)3(ASMA) preparation was excreted unchanged in the urine. The plasma clearance ratio (99mTc(CO)3(ASMA)/131I-OIH) was 81 ± 3 % for D-ASMA compared to only 20 ± 4 % for L-ASMA and 37 ± 7 % for rac-ASMA; the 81 % clearance ratio for D-ASMA isomer is still ∼ 30 % higher than the 99mTc-MAG3/131I-OIH clearance ratio (∼50-60 %). Red cell uptake was similar for all three tracers (6-9 %), and all tracers had a relatively rapid renal excretion; at 3 h, the 99mTc(CO)3(ASMA)/131I-OIH urine ratio was 100 ± 3 % for D-ASMA, 80 ± 2 % for L-ASMA and 88 ± 1 % for rac-ASMA.
Conclusions
The renal excretion characteristics of 99mTc(CO)3(D-ASMA) in humans are superior to those of the other two 99mTc(CO)3(ASMA) isomers studied, but are still inferior to 131I-OIH, even though there was no difference in the clearance of two of 99mTc(CO)3(ASMA) isomers and 131I-OIH in rats. The work described here demonstrates the sensitivity in in vivo biological behavior of 99mTc(CO)3(ASMA) isomers to their subtle structural differences.




Similar content being viewed by others
References
Fritzberg AR, Kasina S, Eshima D, Johnson DL. Synthesis and biological evaluation of technetium-99m MAG3 as a hippuran replacement. J Nucl Med. 1986;27:111–6.
Taylor A, Eshima D, Fritzberg AR, Christian PE, Kasina S. Comparison of iodine-131 OIH and technetium-99m MAG3 renal imaging in volunteers. J Nucl Med. 1986;27:795–803.
Esteves FP, Taylor A, Manatunga A, Folks R, Krishnan M, Garcia EV. 99mTc-MAG3 renography: normal values for MAG3 clearance and curve parameters, excretory parameters and residual urine volume. Am J Roentgenol. 2006;187:W610–7.
Verbruggen AM, Nosco DL, Van Nerom CG, Bormans GM, Adriaens PJ, De Roo MJ. Technetium-99m-L,L-ethylenedicysteine: a renal imaging agent. I. Labeling and evaluation in animals. J Nucl Med. 1992;33:551–7.
Van Nerom CG, Bormans G, Verbruggen AM, Claeys CW, De Roo MJ. Evaluation of 99mTc-L,L-ethylenedicysteine in different species: an overview. In: O’Reilly PH, Taylor A, Nally JV, editors. Radionuclide in Nephrourology. Blue Bell, Pennsylvania, USA: Field and Wood, Medical Periodicals; 1994. p. 13-20.
Taylor A, Hansen L, Eshima D, Malveaux E, Folks R, Shattuck L, et al. Comparison of technetium-99m-LL-EC isomers in rats and humans. J Nucl Med. 1997;38:821–6.
Sanchez J, Friedman S, Kempf J, Abdel-Dayem H. Gallbladder activity appearing 6 minutes after the intravenous injection of Tc99m MAG3 simulating a picture of obstructive uropathy of the right kidney. Clin Nucl Med. 1993;18:30–4.
Shattuck LA, Eshima D, Taylor Jr AT, Anderson TL, Graham DL, Latino FA, et al. Evaluation of the hepatobiliary excretion of technetium-99m-MAG3 and reconstitution factors affecting radiochemical purity. J Nucl Med. 1994;35:349–55.
Rosen JM. Gallbladder uptake simulating hydronephrosis on Tc-99m MAG3 scintigraphy. Clin Nucl Med. 1993;18:713–4.
Gupta NK, Bomanji JB, Waddington W, Lui D, Costa DC, Verbruggen AM, et al. Technetium-99m-L, L-ethylenedicysteine scintigraphy in patients with renal disorders. Eur J Nucl Med. 1995;22:617–24.
Prvulovich EM, Bomanji JB, Waddington WA, Rudrasingham P, Verbruggen AM, Ell PJ. Clinical evaluation of technetium-99m-L, L-ethylenedicysteine in patients with chronic renal failure. J Nucl Med. 1997;38:809–14.
Volkmann R, Friberg P, Jakobsson L, Jensen G, Jonsson B-A, Moonen M. Single-kidney 99mTc-mercaptoacetyltriglycine extraction and clearance as compared with para-aminohippurane. In: O’Reilly PH, Taylor A, Nally JV, editors. Radionuclides in Nephrology. Blue Bell, Pennsylvania: Field and Wood Medical Periodicals, Inc; 1994. p. 21-6.
Jafri RA, Britton KE, Nimmon CC, Solanki K, Al-Nahhas A, Bomanji J, et al. Technetium-99m MAG3, a comparison with iodine-123 and iodine-131 orthoiodohippurate in patients with renal disorders. J Nucl Med. 1988;29:147–58.
Piepsz A, Tondeur M, Kinthaert J, Ham HR. Reproducibility of technetium-99m mercaptoacetyltriglycine clearance. Eur J Nucl Med. 1996;23:195–8.
Klingensmith III WC, Fritzberg AR, Spitzer VM, Johnson DL, Kuni CC, Williamson MR, et al. Clinical evaluation of Tc-99m N, N'-bis(mercaptoacetyl)-2,3-diaminopropanoate as a replacement for I-131 hippurate: concise communication. J Nucl Med. 1984;25:42–8.
Lipowska M, He H, Malveaux E, Xu X, Marzilli LG, Taylor A. First evaluation of a 99mTc-tricarbonyl complex, 99mTc(CO)3(LAN), as a new renal radiopharmaceutical in humans. J Nucl Med. 2006;47:1032–40.
Lipowska M, Marzilli LG, Taylor AT. 99mTc(CO)3-nitrilotriacetic acid: a new renal radiopharmaceutical showing pharmacokinetic properties in rats comparable to those of 131I-OIH. J Nucl Med. 2009;50:454–60.
Taylor AT, Lipowska M, Marzilli LG. 99mTc(CO)3(NTA): a 99mTc renal tracer with pharmacokinetic properties comparable to those of 131I-OIH in healthy volunteers. J Nucl Med. 2010;51:391–6.
Taylor AT, Lipowska M, Cai H. 99mTc(CO)3(NTA) and 131I-OIH: Comparable plasma clearances in patients with chronic kidney disease. J Nucl Med. 2013;54:578–84.
Lipowska M, Klenc J, Marzilli LG, Taylor AT. Preclinical evaluation of 99mTc(CO)3-aspartic-N-monoacetic acid, a renal radiotracer with pharmacokinetic properties comparable to 131I-o-iodohippurate. J Nucl Med. 2012;53:1277–83.
Maderova J, Pavelicik F, Marek J. N-(Carboxymethyl)aspartic acid. Acta Cryst Sect E. 2002;58:o469–70.
Hartman JAR, Woodbury RP, inventors; biodegradable bleach stabilizers for detergents. US patent. 1994;5,362,412.
Snyder RV, Angelici RJ. Stereoselectivity of N-carboxymethyl-amino acid complexes of copper(II) toward optically active amino acids. J Inorg Nucl Chem. 1973;35:523–35.
He H, Lipowska M, Christoforou AM, Marzilli LG, Taylor AT. Initial evaluation of new 99mTc(CO)3 renal imaging agents having carboxyl-rich thioether ligands and chemical characterization of Re(CO)3 analogues. Nucl Med Biol. 2007;34:709–16.
Klenc J, Lipowska M, Taylor AT, Marzilli LG. Synthesis and characterization of fac-Re(CO)3-aspartic-N-monoacetic acid: Structural analogue of a potential renal tracer, fac-99mTc(CO)3(ASMA). Eur J Inorg Chem. 2012:4334-41.
Sapirstein L, Vidt DG, Mandel MJ, Hanusek G. Volumes of distribution and clearance of intravenously injected creatinine in the dog. Am J Physiol. 1955;181:330–6.
Haycock GB, Schwartz GJ, Wisotsky D. Geometric method for measuring body surface area: A height-weight formula validated in infants, children and adults. J Pediatr. 1978;93:62–6.
Russell CD, Thorstad B, Yester MV, Stutzman M, Baker T, Dubovsky EV. Comparison of technetium-99m MAG3 with iodine-131 hippuran by a stimultaneous dual channel technique. J Nucl Med. 1988;29:1189–93.
Eshima D, Fritzberg AR, Taylor A. Tc-99m renal tubular function agents: current status. Semin Nucl Med. 1990;20:28–40.
Eshima D, Eshima L, Hansen L, Lipowska M, Marzilli LG, Taylor A. Effect of protein binding on the renal extraction of I-131 OIH and Tc-99m tubular agents. J Nucl Med. 2000;41:2077–82.
Taylor Jr A, Eshima D. Effects of altered physiologic states on clearance and biodistribution of technetium-99m MAG3, iodine-131 OIH, and iodine-125 iothalamate. J Nucl Med. 1988;29:669–75.
Fritzberg AR, Kuni CC, Klingensmith III WC, Stevens J, Whitney WP. Synthesis and biological evaluation of Tc-99m N, N'-bis-(mercaptoacetyl)-2,3-diaminopropionate: a potential replacement of I-131 hippuran. J Nucl Med. 1982;23:592–8.
Bormans G, Cleynhens B, José D, Hoogmartens M, De Roo M, Verbruggen A. Synthesis and biological characteristics of the four stereoisomers of 99mTc-N, N'-bis-(mercaptoacetyl)-2, 3-diaminopropanoate. Int J Radiat Appl Instrum Part B Nucl Med Biol. 1990;17:499–506.
Taylor AT, Lipowska M, Hansen L, Malveaux E, Marzilli LG. 99mTc-MAEC complexes: new renal radiopharmaceuticals combining characteristics of 99mTc-MAG3 and 99mTc-EC. J Nucl Med. 2004;45:885–91.
Eshima D, Taylor A, Fritzberg AR, Kasina S, Hansen L, Sorenson JF. Animal evaluation of technetium-99m triamide mercaptide complexes as potential renal imaging agents. J Nucl Med. 1987;28:1180–6.
Bormans GM, Cleynhen BJ, De Roo MJ, Verbruggen AM. Evaluation of the renal excretion characterisctics of technetium-99m mercaptoacetylglycyl-D-alanylglycine in healthy volunteers. Eur J Nucl Med. 1992;19:271–7.
Vanbilloen HP, De Roo MJ, Verbruggen AM. Complexes of technetium-99m with tetrapeptides containing one alanyl and three glycyl moieties. Eur J Nucl Med. 1996;23:40–8.
Acknowledgments
This work was funded by the National Institute of Health (NIH/NIDDK) grant R37 DK038842. The authors thank Dr. Liudmila Verdes for her assistance with volunteer recruitment, Eugene Malveaux and Angela Akbasheva for their excellent technical assistance, and our volunteers for their commitment to the studies. Covidien is gratefully acknowledged for providing the IsoLink kits.
Conflict of Interest
Andrew T. Taylor and Russell D. Folks receive royalties from the sale of QuantEM software. This arrangement has been reviewed and approved by Emory University in accordance with its conflict-of-interest policy. Malgorzata Lipowska and Jeffrey Klenc declare that they have no conflict of interest.
Ethics Statement
All studies were performed with the approval the Emory University Institutional Review Board and conducted under the auspices of IND 112,322 from the United States Food and Drug Administration. Written informed consent was obtained from each volunteer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lipowska, M., Klenc, J., Folks, R.D. et al. Initial Evaluation of 99mTc(CO)3(ASMA) as a Renal Tracer in Healthy Human Volunteers. Nucl Med Mol Imaging 48, 216–224 (2014). https://doi.org/10.1007/s13139-014-0270-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-014-0270-8