Abstract
Purpose
The aim of this study was to evaluate the feasibility of FP-CIT PET template-based quantitative analysis on F-18 FP-CIT PET in patients with de novo Parkinson’s disease (PD), compared with MR-based and manual methods. We also assessed the correlation of quantitative parameters of those methods with clinical severity of the disease.
Methods
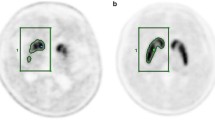
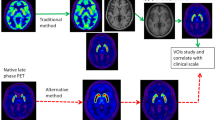
Forty patients with de novo PD underwent both MRI and F-18 FP-CIT PET. Images were spatially normalized to a standardized PET template. Mean counts of 4 ROIs: putamen, caudate, occipital cortex and cerebellum, were obtained using the quantification program, Korean Statistical Probabilistic Anatomical Map (KSPAM). Putamen-to-caudate ratio (PCR), asymmetry index (ASI), specific-to-nonspecific ratios with two different references: to occipital cortex (SOR) and cerebellum (SCR) were compared. Parameters were also calculated from manually drawn ROI method and MR-coregistrated method.
Results
All quantitative parameters showed significant correlations across the three different methods, especially between the PET-based and manual methods. Among them, PET-based SOR and SCR values showed an excellent correlation and concordance with those of manual method. In relationship with clinical severity, only ASI achieved significantly inverse correlations with H&Y stage and UPDRS motor score. There was no significant difference between the quantitative parameters of both occipital cortex and cerebellum in all three methods, which implied that quantitation using PET-based method could be reproducible regardless of the reference region.
Conclusions
Quantitative parameters using FP-CIT PET template-based method correlated well with those using laborious manual method with excellent concordance. Moreover, PET-based quantitation was less influenced by the reference region than MR-based method. It suggests that PET-based method can provide objective and quantitative parameters quickly and easily as a feasible analysis in place of conventional method.




Similar content being viewed by others
References
Ma Y, Dhawan V, Mentis M, Chaly T, Spetsieris PG, Eidelberg D. Parametric mapping of [18F]FPCIT binding in early stage Parkinson’s disease: a PET study. Synapse. 2002;45:125–33.
Kazumata K, Dhawan V, Chaly T, Antonini A, Margouleff C, Belakhlef A, et al. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med. 1998;39:1521–30.
Wang J, Zuo CT, Jiang YP, Guan YH, Chen ZP, Xiang JD, et al. 18F-FP-CIT PET imaging and SPM analysis of dopamine transporters in Parkinson’s disease in various Hoehn & Yahr stages. J Neurol. 2007;254:185–90.
Oh M, Kim JS, Kim JY, Shin KH, Park SH, Kim HO, et al. Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med. 2012;53:399–406.
Oh JK. Clinical significance of F-18 FP-CIT dual time point pet imaging in idiopathic Parkinson’s disease. Nucl Med Mol Imaging. 2011;45:255–60.
Chun HJ. Clinical significance of 123I-IPT SPECT for the diagnosis of the Parkinson’s disease. J Korean Neurosurg Soc. 2003;34:104–9.
Benamer TS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord. 2000;15:503–10.
Acton PD, Newberg A, Plossl K, Mozley PD. Comparison of region-of-interest analysis and human observers in the diagnosis of Parkinson’s disease using [99mTc]TRODAT-1 and SPECT. Phys Med Biol. 2006;51:575–85.
Ortega Lozano SJ, Martinez Del Valle Torres MD, Ramos Moreno E, Sanz Viedma S, Amrani Raissouni T, Jimenez-Hoyuela JM. Quantitative evaluation of SPECT with FP-CIT. Importance of the reference area. Rev Esp Med Nucl. 2010;29:246–50.
Tossici-Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS. Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006;33:1491–9.
Filippi L, Bruni C, Padovano F, Schillaci O, Simonetti G. The value of semi-quantitative analysis of 123I-FP-CIT SPECT in evaluating patients with Parkinson’s disease. Neuroradiol J. 2008;21:505.
Kim BS. The discriminating nature of dopamine transporter image in Parkinsonism: the competency of dopaminergic transporter imaging in differential diagnosis of Parkinsonism: 123I-FP-CIT SPECT study. Nucl Med Mol Imaging. 2007;41:272–9.
Lee JS. Quantification of brain images using Korean standard templates and structural and cytoarchitectonic probabilistic maps. Korean J Nucl Med. 2004;38:241–52.
Koo BB. Developing a Korean standard brain atlas on the basis of statistical and probabilistic approach and visualization tool for functional image analysis. Korean J Nucl Med. 2003;37:162–70.
Lee JS, Lee DS. Analysis of functional brain images using population-based probabilistic atlas. Curr Med Imaging Rev. 2005;1:81–7.
Scherfler C, Seppi K, Donnemiller E, Goebel G, Brenneis C, Virgolini I, et al. Voxel-wise analysis of [123I]beta-CIT SPECT differentiates the Parkinson variant of multiple system atrophy from idiopathic Parkinson’s disease. Brain. 2005;128(Pt 7):1605–12.
Choi JY, Lee KH, Na DL, Byun HS, Lee SJ, Kim H, et al. Subcortical aphasia after striatocapsular infarction: quantitative analysis of brain perfusion SPECT using statistical parametric mapping and a statistical probabilistic anatomic map. J Nucl Med. 2007;48:194–200.
Lee TH, Kim SJ, Kim IJ, Kim YK, Kim DS, Park KP. Statistical parametric mapping and statistical probabilistic anatomical mapping analyses of basal/acetazolamide Tc-99 m ECD brain SPECT for efficacy assessment of endovascular stent placement for middle cerebral artery stenosis. Neuroradiol. 2007;49:289–98.
Lee DS, Lee JS, Kang KW, Jang MJ, Lee SK, Chung JK, et al. Disparity of perfusion and glucose metabolism of epileptogenic zones in temporal lobe epilepsy demonstrated by SPM/SPAM analysis on 15O water PET, [18F]FDG-PET, and [99mTc]-HMPAO SPECT. Epilepsia. 2001;42:1515–22.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42.
Fahn S, Elton R, Committee. MotUD. The unified Parkinson’s disease rating scale. In: Fahn SMC, Calne DB, Goldstein M, editors. Recent developments in Parkinson’s disease. Florham Park: Macmillan Healthcare Information; 1987. p. 154–63. pp293–304.
Lee SJ, Oh SJ, Chi DY, Kang SH, Kil HS, Kim JS, et al. One-step high-radiochemical-yield synthesis of [18F]FP-CIT using a protic solvent system. Nucl Med Biol. 2007;34:345–51.
Lee SJ, Oh SJ, Moon WY, Choi MS, Kim JS, Chi DY, et al. New automated synthesis of [18F]FP-CIT with base amount control affording high and stable radiochemical yield: a 1.5-year production report. Nucl Med Biol. 2011;38:593–7.
Lee JS, Park KS, Lee DS, Lee CW, Chung JK, Lee MC. Development and applications of a software for Functional Image Registration (FIRE). Comput Methods Programs Biomed. 2005;78:157–64.
Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988.
Takada S, Yoshimura M, Shindo H, Saito K, Koizumi K, Utsumi H, et al. New semiquantitative assessment of 123I-FP-CIT by an anatomical standardization method. Ann Nucl Med. 2006;20:477–84.
Calvini P, Rodriguez G, Inguglia F, Mignone A, Guerra UP, Nobili F. The basal ganglia matching tools package for striatal uptake semi-quantification: description and validation. Eur J Nucl Med Mol Imaging. 2007;34:1240–53.
Ishikawa T, Dhawan V, Kazumata K, Chaly T, Mandel F, Neumeyer J, et al. Comparative nigrostriatal dopaminergic imaging with iodine-123-beta CIT-FP/SPECT and fluorine-18-FDOPA/PET. J Nucl Med. 1996;37:1760–5.
Asenbaum S, Brücke T, Pirker W, Podreka I, Angelberger P, Wenger S, et al. Imaging of dopamine transporters with iodine-123-beta-CIT and SPECT in Parkinson’s disease. J Nucl Med. 1997;38:1–6.
Rinne JO, Ruottinen H, Bergman J, Haaparanta M, Sonninen P, Solin O. Usefulness of a dopamine transporter PET ligand [18F]β-CFT in assessing disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:737–41.
Hubbuch M, Farmakis G, Schaefer A, Behnke S, Schneider S, Hellwig D, et al. FP-CIT SPECT does not predict the progression of motor symptoms in Parkinson’s disease. Eur Neurol. 2011;65:187–92.
Acknowledgments
This study was partly supported by Korea University Research Special Grants (2011-K1131781, 2011-K1132941). We had no conflicts of interest relevant to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, E., Oh, S.Y., Pahk, K. et al. Feasibility of PET Template-Based Analysis on F-18 FP-CIT PET in Patients with De Novo Parkinson’s Disease. Nucl Med Mol Imaging 47, 73–80 (2013). https://doi.org/10.1007/s13139-013-0196-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-013-0196-6