Abstract
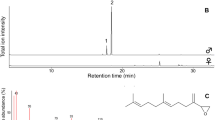
Chemical communication in the family Hesperiidae (Lepidoptera) is practically unstudied, even though this group includes approximately 4,000 species and represents a fifth of the world’s butterfly fauna. We present the first comparative morphological and chemical analysis of scent organs for nine species in the genus Pyrgus, the most species-rich hesperiid genus in the Palearctic region. Our results show that the morphology of the two main male scent organs—the costal fold and the tibial tufts—does not differ between species. The chemical analyses detected a total of 125 different compounds exclusively present in these organs. We document great interspecific differences and much narrower intraspecific variability in the chemical profiles. The dynamics of chemical versus genetic distances indicate two different phases: a faster (but more variable) initial chemical divergence at lower genetic divergences (probably related to speciation) and a slower but more constant differentiation (drift). As a result most species can be identified based on their chemical profiles, except for a closely related species pair (P. malvae/P. malvoides) for which hybridisation is common in the contact zone. Our results suggest that the Hesperiidae is a group with great potential for the study of chemical communication that deserves further attention.









Similar content being viewed by others
References
Aistleitner, E. (1996). Die Arealgrenzen der beiden Dickkopffalter-Arten Pyrgus malvae L. und Pyrgus malvoides Elw. & Edw. (Lepidoptera Hesperiidae) in Vorarlberg (Österreich) und Liechtenstein. Vorarlb Natursch, 1, 335–344.
Andersson, J., Borg-Karlson, A.-K., & Wiklund, C. (2003). Antiaphrodisiacs in pierid butterflies: a theme with variation! Journal of Chemical Ecology, 29, 1489–1499.
Andersson, J., Borg-Karlson, A.-K., Vongvanich, N., & Wiklundf, C. (2007). Male sex pheromone release and female mate choice in a butterfly. The Journal of Experimental Biology, 210, 964–970.
Ando, T., Inomata, S., & Yamamoto, M. (2004). Lepidopteran sex pheromones. In S. Schulz (Ed.), The chemistry of pheromones and other semiochemicals. Topics in Current Chemistry 239 (pp. 51–96). Berlin: Springer.
Baker, T. C., Nishida, R., & Roelofs, W. L. (1981). Close-range attraction of female oriental fruit moths to herbal scent of male hairpencils. Science, 214, 1359–1361.
Berlocher, S. H., & Feder, J. L. (2002). Sympatric speciation in phytophagous insects: moving beyond controversy? Annual Review of Entomology, 47, 773–815.
Birch, M. C. (1970a). Structure and function of the pheromone-producing brush-organs in males of Phlogophora meticulosa (L.) (Lepidoptera: Noctuidae). Transactions of the Royal Entomological Society of London, 122, 277–292.
Birch, M. C. (1970b). Pre-courtship use of abdominal brushes by the nocturnal moth, Phlogophora meticulosa (L.) (Lepidoptera: Noctuidae). Animal Behaviour, 18, 310–316.
Birch, M. C. (1975). Aphrodisiac pheromones in insects. In M. C. Birch (Ed.), Pheromones (pp. 115–134). New York: Elsevier.
Birch, M. C., & Hefetz, A. (1987). Extrusible organs in male moths and their role in courtship behavior. Bulletin of the Entomological Society of America, 33, 222–229.
Birch, M. C., Poppy, G. M., & Baker, T. C. (1990). Scents and eversible scent structures of male moths. Annual Review of Entomology, 35, 25–58.
Boppré, B. (1989). Chemically mediated interactions between butterflies. In R. I. Vane-Wright & P. R. Ackery (Eds.), The biology of butterflies (pp. 259–275). Princeton: Princeton University Press.
Boppré, B. (1993). The American monarch: courtship and chemical communication of a peculiar danaine butterfly. In S. B. Malcolm & M. P. Zalucki (Eds.), Biology and conservation of the monarch butterfly (pp. 29–41). Los Angeles: Natural History Museum of Los Angeles County.
Bridges, C. A. (1994). Catalogue of the Family-group, Genus-group and Species-group Names of the Hesperiidae (Lepidoptera) of the World. Illinois: Urbana III.
Coyne, J. A., & Orr, H. A. (1989). Two rules of speciation. In D. Otte & J. Ender (Eds.), Speciation and its consequences (pp. 180–207). Sunderland: Sinauer Associates Inc.
Coyne, J. A., & Orr, H. A. (2004). Speciation. Sunderland: Sinauer Associates Inc.
Dapporto, L. (2007). Cuticular lipid diversification in Lasiommata megera and L. paramegera: the influence of species, sex, and population (Lepidoptera, Nymphalidae). Biological Journal of the Linnean Society, 91, 703–710.
De Jong, R. (1972). Systematics and geographic history of the genus Pyrgus in the palaearctic region. Tijdschrift voor Entomologie, 115, 1–121.
De Jong, R. (1982). Secondary sexual characters in Celaenorrhinus and the delimitation of the genus (Lepidoptera, Hesperiidae). Journal of Natural History, 16, 695–705.
Dyar, H. G. (1905). A review of the Hesperiidae of the United States. Journal of the New York Entomological Society, 13, 111–141.
El-Sayed, A. M. (2009). The Pherobase: Database of Insect Pheromones and Semiochemicals. Available: http://www.pherobase.com. Accessed 2012 Jan 24.
Ferreira, T. A. & Rasband, W. (2010). The ImageJ User Guide, Version 1.43. Avalaible: http://rsbweb.nih.gov/ij/docs/user-guide.pdf/. Accessed 2012 Apr 25.
Fitzpatrick, S. M., & McNeil, J. N. (1988). Male scent in lepidopteran communication: the role of male pheromone in mating behaviour of Pseudaletia unipuncta (Haw.) (Lepidoptera: Noctuidae). Memoirs of the Entomological Society of Canada, 146, 131–151.
García-Barros, E., & Meneguz, M. (2012). Estructura de las escamas ventrales de las alas de Coenonympha Hübner, [1819], con especial referencia a C. pamphilus (L., 1758) y su morfotipo lyllus (Esper, 1805) (Lepidoptera: Nymphalidae). SHILAP Revista de lepidopterología, 40(159), 279–293.
Grichanov, I. Y. (1998). Lepidoptera male pheromones in relation to the origin of the pheromone system in insects. Russian Entomological Journal, 7, 77–82.
Groot, A. T., Horovitz, J. L., Hamilton, J., Santangelo, R. G., Schal, C., & Gould, F. (2006). Experimental evidence for interspecific directional selection on moth pheromone communication. Proceedings of the National Academy of Sciences of the United States of America, 103, 5858–5863.
Guillaumin, M. (1963). Le complexe odoriférant thoraco-abdominal de Pyrgus malvae L. (Lep. Hesperiidae). Bulletin de la Societe Entomologique de France, 68, 128–136.
Heath, R. R., Landolt, P. J., Dueben, B. D., Murphy, R. E., & Schneider, R. E. (1992). Identification of male cabbage looper sex pheromone attractive to females. Journal of Chemical Ecology, 18, 441–453.
Hebert, P. D. N., Penton, E. H., Burns, J. M., Janzen, D. H., & Hallwachs, W. (2004). Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proceedings of the National Academy of Sciences of the United States of America, 101, 14812–14817.
Hernández-Roldán, J. L. (2005). Pyrgus bellieri (Oberthür, 1910) en España. Estudio morfométrico comparativo con Pyrgus alveus (Hübner, [1803]) (Lepidoptera: Hesperiidae). Minor thesis dissertation, Universidad Autónoma de Madrid.
Huang, Y., Xu, S., Tang, X., Zhao, Z., & Du, J. (1996). Male orientation inhibitor of cotton bollworm: identification of compounds produced by male hairpencil glands. Entomologia Sinica, 3, 172–182.
Kirkpatrik, M., & Ravigné, V. (2002). Speciation by natural and sexual selection: models and experiments. American Naturalist, 159, 22–35.
LSPN. (1999). Les papillons et leurs biotopes. Egg: Fotorotar AG.
Nishida, R., Schulz, S., Kim, C. S., Fukami, H., Kuwahara, Y., Honda, K., & Hayashi, N. (1996). Male sex pheromone of a giant danaine butterfly, Idea leuconoe. Journal of Chemical Ecology, 22, 949–972.
Ômura, H., & Honda, H. (2011). Pungent odor of the adult skipper butterfly Erynnis montanus (Lepidoptera: Hesperiidae). Applied Entomology and Zoology, 46, 281–286.
Phelan, P. L., Silk, P. J., Northcott, C. J., Tan, S. H., & Baker, T. C. (1986). Chemical identification and behavioral characterization of male wing pheromone of Ephesia elutella (Pyralidae). Journal of Chemical Ecology, 12, 135–146.
Pivnick, K. A., Lavoie-Dornik, J., & McNeil, J. N. (1992). The role of the androconia in the mating behaviour of the European skipper, Thymelicus lineola, and evidence for a male sex pheromone. Physiological Entomology, 17, 260–268.
Ritchie, M. G. (2007). Sexual selection and speciation. Annual Review of Ecology, Evolution, and Systematics, 38, 79–102.
Robbins, R. K. (1982). How many butterfly species? The News of the Lepidopterists´ Society, 41, 214–216.
Roelofs, W. L., & Brown, R. L. (1982). Pheromones and evolutionary relationships of Tortricidae. Annual Review of Ecology and Systematics, 13, 395–422.
Roelofs, W. L., & Rooney, A. P. (2003). Molecular genetics and evolution of pheromone biosynthesis in Lepidoptera. Proceedings of the National Academy of Sciences of the United States of America, 100, 9179–9184.
Schulz, S., Franche, W., & Boppré, M. (1988). Carboxylic acids from hairpencils of male Amauris butterflies (Lep.: Danainae). Biological Chemistry Hoppe Seyler, 369, 633–638.
Schulz, S., Beccaloni, G., Brown, K. S., Boppré, M., Freitas, A. V. L., Ockenfels, P., & Trigo, J. R. (2004). Semiochemicals derived from pyrrolizidine alkaloids in male ithomiine butterflies (Lepidoptera: Nymphalidae: Ithomiinae). Biochemical Systematics and Ecology, 32, 699–713.
Singh, R. S., & Kulathinal, R. J. (2000). Sex gene pool evolution and speciation: A new paradigm. Genes & Genetic Systems, 75, 119–130.
Smadja, C., & Butlin, R. K. (2009). On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity, 102, 77–97.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739.
Teal, P. E. A., & Tumlinson, J. H. (1989). Isolation, identification and biosynthesis of compounds produced by male hairpencil glands of Heliothis virescens (F.) (Lepidoptera: Lepidoptera). Journal of Chemical Ecology, 15, 413–427.
Templeton, A. R. (1981). Mechanisms of speciation - A population genetic approach. Annual Review of Ecology, Evolution, and Systematics, 12, 23–48.
Thibout, E., Ferary, S., & Auger, J. (1994). Nature and role of the sexual pheromones emitted by males of Acrolepiopsis assectella. Journal of Chemical Ecology, 20, 1571–1581.
Warren, A. D. (2006). The higher classification of Hesperiidae (Lepidoptera: Hesperioidea). PhD dissertation, Oregon State University. Available: http://gradworks.umi.com/32/19/3219773.html. Accessed 2012 Jan 26.
Warren, A. D., Ogawa, J. R., & Brower, A. V. Z. (2008). Phylogenetic relationships of subfamilies and circumscription of tribes in the family Hesperiidae (Lepidoptera: Hesperioidea). Cladistics, 24, 642–676.
Warren, A. D., Ogawa, J. R., & Brower, A. V. Z. (2009). Revised classification of the Hesperiidae (Lepidoptera: Hesperioidea) based on combined molecular and morphological data. Systematic Entomology, 34, 467–523.
Witzgall, P., Lindblom, T., Bengtsson, & Tóth, M. (2004). The Pherolist. Available: http://www-pherolist.slu.se. Accessed 2012 Jan 24.
Wuest, J. (1998). The pheromone dispersing apparatus in some Hesperiinae (Lepidoptera: Hesperiidae). Swiss Journal of Zoology, 105, 813–822.
Wyatt, T. D. (2003). Pheromones and Animal Behaviour. Communication by Smell and Taste. Cambridge: Cambridge University Press.
Acknowledgments
We thank Vlad Dincă (Stockholm University, Stockholm, Sweden, and Institut de Biologia Evolutiva CSIC-UPF, Barcelona, Spain) for his help in obtaining samples and genetic analyses. We are also grateful to Rosa Sedano (Laboratory GC-LC, SIDI from Universidad Autónoma de Madrid, Spain) for performing the chromatography analyses and to Esperanza Salvador, Enrique Rodríguez, and Isidoro Poveda (Laboratory SEM-EDX, SIDI from Universidad Autónoma de Madrid, Spain) for help with scanning electron microscope images. Andrew Warren (McGuire Center for Lepidoptera and Biodiversity, University of Florida, USA) very kindly shared his knowledge on Hesperiidae with us. Funding for this research was provided by Universitat Autònoma de Barcelona (project EME2007-33 to J.L.H.-R., R.B. and R.V.) and by the Spanish Ministerio de Ciencia e Innovación (project CGL2010-21226/BOS to J.L.H.-R. and R.V. and project CGL2004-04680-c10-08/BOS to M.L.M.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 5.30 mb)
Rights and permissions
About this article
Cite this article
Hernández-Roldán, J.L., Bofill, R., Dapporto, L. et al. Morphological and chemical analysis of male scent organs in the butterfly genus Pyrgus (Lepidoptera: Hesperiidae). Org Divers Evol 14, 269–278 (2014). https://doi.org/10.1007/s13127-014-0170-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-014-0170-x