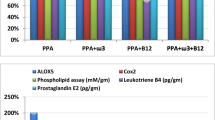
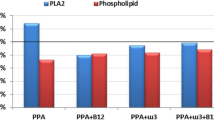
Abstract
The present study was undertaken to elucidate the effect of alpha-linolenic acid (ALA, 18:3, ω-3) and gamma-linolenic acid (GLA, 18:3, ω-6) on experimental autism features induced by early prenatal exposure to valproic acid (VPA) in albino wistar pups. The pups were scrutinized on the accounts of behavioral, biochemical, and inflammatory markers, and the results suggested that the GLA can impart significant protection in comparison to ALA against VPA-induced autism features. When scrutinized histopathologically, the cerebellum of the GLA-treated animals was evident for more marked protection toward neuronal degeneration and neuronal loss in comparison to ALA. Concomitant administration of ALA and GLA with VPA demonstrated a marked cutdown in the Pgp 9.5 expression with GLA having more pronounced effect. Henceforth, it can be concluded that ALA and GLA can impart favorable protection against the VPA-induced autism-like features with GLA having pronounced effect.






Similar content being viewed by others
References
Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L (2009) Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem 42:1032–1040
Altman J, Sudarshan K (1975) Postnatal development of locomotion in the laboratory rat. Anim Behav 23:896–920
Anand R, Kaithwas G (2014) Anti-inflammatory potential of alpha-linolenic acid mediated through selective COX inhibition: computational and experimental data. Inflammation 37:1297–1306
Bambini-Junior V, Rodrigues L, Behr GA, Moreira JCF, Riesgo R, Gottfried C (2011) Animal model of autism induced by prenatal exposure to valproate: behavioral changes and liver parameters. Brain Res 1408:8–16
Bauman M, Kemper TL (1985) Histoanatomic observations of the brain in early infantile autism. Neurology 35:866–866
Bauman ML, Kemper TL (2005) Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci 23:183–187
Belch JJ, Hill A (2000) Evening primrose oil and borage oil in rheumatologic conditions. Am J Clin Nutr 71:352s–356s
Belur B, Kandaswamy N, Mukherjee K (1990) Laboratory techniques in histopathology. Medical laboratory technology
Bent S, Bertoglio K, Ashwood P, Bostrom A, Hendren RL (2011) A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder. J Autism Dev Disord 41:545–554
Bhosale UA, Yegnanarayan R, Pophale PD, Zambare MR, Somani RS (2011) Study of central nervous system depressant and behavioral activity of an ethanol extract of Achyranthes aspera (Agadha) in different animal models. International Journal of Applied and Basic Medical Research 1:104
Bilguvar K, Tyagi NK, Ozkara C, Tuysuz B, Bakircioglu M, Choi M, Delil S, Caglayan AO, Baranoski JF, Erturk O (2013) Recessive loss of function of the neuronal ubiquitin hydrolase UCHL1 leads to early-onset progressive neurodegeneration. Proc Natl Acad Sci 110:3489–3494
Bourre J, Dumont O, Piciotti M, Clement M, Chaudiere J, Bonneil M, Nalbone G, Lafont H, Pascal G, Durand G (1991) Essentiality of ω3 fatty acids for brain structure and function1. In: health effects of omega 3 polyunsaturated fatty acids in seafoods. Karger Publishers, pp 103–117
Covington MB (2004) Omega-3 fatty acids. Atlantic 1:2.0
Crane FL, Low H, Sun IL (2012) Evidence for a relation between plasma membrane coenzyme Q and autism. Frontiers in bioscience (Elite edition) 5:1011–1016
Croen LA, Grether JK, Hoogstrate J, Selvin S (2002) The changing prevalence of autism in California. J Autism Dev Disord 32:207–215
Cullen L, Kelly L, Connor SO, Fitzgerald DJ (1998) Selective cyclooxygenase-2 inhibition by nimesulide in man. J Pharmacol Exp Ther 287:578–582
Daynes RA, Jones DC (2002) Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol 2:748–759
Depino AM (2013) Peripheral and central inflammation in autism spectrum disorders. Mol Cell Neurosci 53:69–76
El-Ansary AK, Bacha AGB, Al-Ayadhi LY (2011) Impaired plasma phospholipids and relative amounts of essential polyunsaturated fatty acids in autistic patients from Saudi Arabia. Lipids Health Dis 10:1
Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, Montiel-Nava C, Patel V, Paula CS, Wang C (2012) Global prevalence of autism and other pervasive developmental disorders. Autism Res 5:160–179
Folch J, Lees M, Sloane-Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O (2006) Ubiquitin hydrolase Uch-L1 rescues β-amyloid-induced decreases in synaptic function and contextual memory. Cell 126:775–788
Haas H, Schauenstein K (2001) Immunity, hormones, and the brain. Allergy 56:470–477
Kaithwas G, Majumdar DK (2012) In vitro antioxidant and in vivo antidiabetic, antihyperlipidemic activity of linseed oil against streptozotocin-induced toxicity in albino rats. Eur J Lipid Sci Technol 114:1237–1245
Kaithwas G, Dubey K, Bhatia D, Sharma AD, Pillai K (2007) Reversal of sodium nitrite induced impairment of spontaneous alteration by Aloe vera gel: involvement of cholinergic system. Pharmacologyonline 3:428–437
Kaithwas G, Mukerjee A, Kumar P, Majumdar DK (2011a) Linum usitatissimum (linseed/flaxseed) fixed oil: antimicrobial activity and efficacy in bovine mastitis. Inflammopharmacology 19:45–52
Kaithwas G, Mukherjee A, Chaurasia A, Majumdar DK (2011b) Antiinflammatory, analgesic and antipyretic activities of Linum usitatissimum L. (flaxseed/linseed) fixed oil. Indian J Exp Biol 49(12):932–938
Kapoor R, Huang Y-S (2006) Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol 7:531–534
Karvat G, Kimchi T (2014) Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology 39:831–840
Kern JK, Geier DA, Sykes LK, Geier MR (2013) Evidence of neurodegeneration in autism spectrum disorder. Translational neurodegeneration 2:1
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lu W, Zhao X, Xu Z, Dong N, Zou S, Shen X, Huang J (2013) Development of a new colorimetric assay for lipoxygenase activity. Anal Biochem 441:162–168
Lyall K, Munger KL, O'Reilly ÉJ, Santangelo SL, Ascherio A (2013) Maternal dietary fat intake in association with autism spectrum disorders. Am J Epidemiol :kws433
Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, Shago M, Moessner R, Pinto D, Ren Y (2008) Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet 82:477–488
Meiri G, Bichovsky Y, Belmaker R (2009) Omega 3 fatty acid treatment in autism. Journal of child and adolescent psychopharmacology 19:449–451
Mosconi MW, Wang Z, Schmitt LM, Tsai P, Sweeney JA (2015) The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders. Front Neurosci 9
Nader R, Oberlander TF, Chambers CT, Craig KD (2004) Expression of pain in children with autism. Clin J Pain 20:88–97
Olexová L, Senko T, Štefánik P, Talarovičová A, Kršková L (2013) Habituation of exploratory behaviour in VPA rats: animal model of autism. Interdiscip Toxicol 6:222–227
Poling JS, Frye RE, Shoffner J, Zimmerman AW (2006) Developmental regression and mitochondrial dysfunction in a child with autism. J Child Neurol 21:170–172
Raj P, Singh M, Rawat JK, Gautam S, Saraf SA, Kaithwas G (2014) Effect of enteral administration of α-linolenic acid and linoleic acid against methotrexate induced intestinal toxicity in albino rats. RSC Adv 4:60397–60403
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Riendeau D, Percival M, Brideau C, Charleson S, Dube D, Ethier D, Falgueyret J-P, Friesen R, Gordon R, Greig G (2001) Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther 296:558–566
Rolf L, Haarmann F, Grotemeyer KH, Kehrer H (1993) Serotonin and amino acid content in platelets of autistic children. Acta Psychiatr Scand 87:312–316
Rose S, Frye RE, Slattery J, Wynne R, Tippett M, Pavliv O, Melnyk S, James SJ (2014) Oxidative stress induces mitochondrial dysfunction in a subset of autism lymphoblastoid cell lines in a well-matched case control cohort. PLoS One 9:e85436
Rossi R, Tsikas D (2009) S-Nitrosothiols in blood: does photosensitivity explain a 4-order-of-magnitude concentration range? Clin Chem 55:1036–1038
Schapiro S, Salas M, Vukovich K (1970) Hormonal effects on ontogeny of swimming ability in the rat: assessment of central nervous system development. Science 168:147–151
Schneider T, Przewłocki R (2005) Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30:80–89
Sharma N, Ahmad Y (2011) An effective method for the analysis of human plasma proteome using two-dimensional gel electrophoresis. Journal of Proteomics & Bioinformatics 2009
Simopoulos AP (1991) Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr 54:438–463
Sliwinski S, Croonenberghs J, Christophe A, Deboutte D, Maes M (2006) Polyunsaturated fatty acids: do they have a role in the pathophysiology of autism? Neuro endocrinology letters 27:465–471
Souza A, Dussan-Sarria JA, Medeiros LF, Souza AC, Oliveira C, Scarabelot VL, Adachi LN, Winkelmann-Duarte EC, Philippi-Martins BB, Netto CA (2014) Neonatal hypoxic–ischemic encephalopathy reduces c-Fos activation in the rat hippocampus: evidence of a long-lasting effect. Int J Dev Neurosci 38:213–222
Spencer L, Mann C, Metcalfe M, Webb MB, Pollard C, Spencer D, Berry D, Steward W, Dennison A (2009) The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer 45:2077–2086
Teitelbaum JE, Walker WA (2001) Review: the role of omega 3 fatty acids in intestinal inflammation. J Nutr Biochem 12:21–32
Theoharides TC, Asadi S, Patel AB (2013) Focal brain inflammation and autism. J Neuroinflammation 10:1
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci 76:4350–4354
Weidenheim KM (2001) Neurobiology of autism: an update. Salud Mental 24:3–9
Wenning GK, Jellinger KA (2005) The role of α-synuclein in the pathogenesis of multiple system atrophy. Acta Neuropathol 109:129–140
Zoroglu SS, Armutcu F, Ozen S, Gurel A, Sivasli E, Yetkin O, Meram I (2004) Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism. Eur Arch Psychiatry Clin Neurosci 254:143–147
Acknowledgments
The authors would like to thank the University Grants Commission for providing fellowship to MS, JKR, SG, and RKY and would also like to thank to the Department of Science and Technology for providing fellowship to SR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Sneha Yadav and Virendra Tiwari have equal contribution.
Electronic supplementary material
Supplementary Figure 1
FAME of the brain tissue subjected to ALA and GLA. Group I: control (3 ml/kg), group II: positive control (400 mg/kg), group III: ALA(3 ml/kg), group IV: GLA(3 ml/kg), group V: VPA + ALA(400 mg/kg + 3 ml/kg), and group VI:VPA + GLA(400 mg/kg + 3 ml/kg) (DOCX 106 kb)
Rights and permissions
About this article
Cite this article
Yadav, S., Tiwari, V., Singh, M. et al. Comparative efficacy of alpha-linolenic acid and gamma-linolenic acid to attenuate valproic acid-induced autism-like features. J Physiol Biochem 73, 187–198 (2017). https://doi.org/10.1007/s13105-016-0532-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-016-0532-2