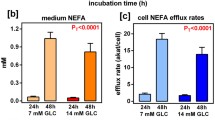
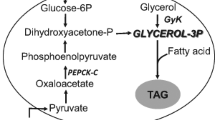
Abstract
Adipose tissue normally has low glycerol kinase activity, but its expression is enhanced under conditions of augmented insulin sensitivity and/or obesity. Since these conditions occur during early pregnancy, the comparative utilization of glucose or glycerol by isolated adipocytes from rats at 0, 7, 14, or 20 days of pregnancy was studied. Incubations were carried out in the presence of [U14C]-glucose or -glycerol in medium supplemented or not with 5 mM glucose and 100 nM insulin. The conversion of glucose into esterified fatty acids and glyceride glycerol was greatest in adipocytes from 7-day pregnant rats, the effect being further enhanced by insulin. Both the amount of aquoporin 7 and the in vitro conversion of glycerol into glyceride glycerol were greatest in adipocytes of 7-day pregnant rats, the later being unaltered by insulin. In the presence of glucose, the overall glycerol utilization was lower than in its absence and glycerol conversion into glyceride glycerol was further decreased by insulin, the effect only being significant in adipocytes from 7-day pregnant rats. It is proposed that the enhanced utilization of glycerol for glyceride glycerol synthesis in adipose tissue contributes to the net accumulation of fat depots that normally takes place in early pregnancy.




Similar content being viewed by others
References
Bellido J, Herrera E (1978) Effects of glucose on the metabolization of fructose and glycerol by isolated adipocytes from rat. Rev Esp Fisiol 34:437–442
Bellido J, Herrera E (1978) Utilization of pyruvate, alanine and glutamate by isolated fat cells and their effects on glycerol metabolism. Rev Esp Fisiol 34:429–436
Brownsey RW, Boone AN, Elliott JE, Kulpa JE, Lee WM (2006) Regulation of acetyl-CoA carboxylase. Biochem Soc Trans 34:223–227
Chaves JM, Herrera E (1980) In vitro response of glycerol metabolism to insulin and adrenalin in adipose tissue from fed and fasted rats during pregnancy. Biol Neonate 38:139–145
Dole VP, Meinertz H (1960) Microdetermination of long chain fatty acids in plasma and tissues. J Biol Chem 235:2595–2599
Dolnikoff M, Martín-Hidalgo A, Machado UF, Lima FB, Herrera E (2001) Decreased lipolysis and enhanced glycerol and glucose utilization by adipose tissue prior to development of obesity in monosodium glutamate (MSG) treated-rats. Int J Obes 25:426–433
Domínguez MC, Herrera E (1976) Effects of glycerol and glucose on the kinetics of glycerol utilization by adipose tissue in the rat. Rev Esp Fisiol 32:293–300
Domínguez MC, Herrera E (1976) The effect of glucose, insulin and adrenaline on glycerol metabolism "in vitro" in rat adipose tissue. Biochem J 158:183–190
Domínguez MC, Herrera E (1976) Effects of 2-deoxy-D-glucose, oligomycin and theophylline on in vitro glycerol metabolism in rat adipose tissue: response to insulin and epinephrine. Horm Metabol Res 8:33–37
Elliott JA (1975) The effect of pregnancy on the control of lipolysis in fat cells isolated from human adipose tissue. Eur J Clin Invest 5:159–163
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 22:24–36
Freinkel N (1980) Banting lecture 1980. Of pregnancy and progeny. Diabetes 29:1023–1035
Guan HP, Li Y, Jensen MV, Negard CB, Steppan CM, Lazar MA (2002) A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat Med 8:1122–1128
Halestrap AP, Denton M (1973) Insulin and the regulation of adipose-tissue acetyl-CoA carboxylase. Biochem J 132:509–517
Hanson RW, Reshef L (2003) Glyceroneogenesis revisited. Biochimie 85:1199–1205
Herrera E, Amusquivar E, Cacho J (2000) Changes in dietary fatty acids modify the decreased lipolytic β3-adrenergic response to hyperinsulinemia in adipocytes from pregnant and nonpregnant rats. Metabolism 49:1180–1187
Herrera E, Ayanz A (1972) Calculation of lipolysis and esterification from glycerol metabolism in rat adipose tissue. J Lipid Res 13:802–809
Herrera E, Lamas L (1970) Utilization of glycerol by rat adipose tissue in vitro. Biochem J 120:433–434
Herrera E, Lasunción MA, Gomez Coronado D, Aranda P, Lopez Luna P, Maier I (1988) Role of lipoprotein lipase activity on lipoprotein metabolism and the fate of circulating triglycerides in pregnancy. Am J Obstet Gynecol 158:1575–1583
Herrera E, Lasunción MA, Martín A, Zorzano A (1992) Carbohydrate-lipid interactions in pregnancy. In: Herrera E, Knopp RH (eds) Perinatal biochemistry. CRC Press, Boca Raton, pp 1–18
Hibuse T, Maeda N, Funahashi T, Yamamoto K, Nagasawa A, Mizunoya W, Kishida K, Inoue K, Kuriyama H, Nakamura T, Fushiki T, Kihara S, Shimomura I (2005) Aquaporin 7 deficiency is associated with development of obesity through activation of adipose tissue glycerol kinase. PNAS 102:10993–10998
Ho R-J, Fan C-C, Barrera LA (1979) Comparison of adipose tissue glycerol kinase of hyperglycemic obese mice and lean litter-mates. Mol Cell Biochem 27:89–96
Hubbard RW, Voorheis HP, Therriault DG (1970) The incorporation of glycerol into the glyceride-glycerol of fat cells isolated from chronically cold-exposed rats. Lipids 5:114–120
Hytten FE, Leitch I (1971) The physiology of human pregnancy. Blackwell Scient Publisher, Oxford
Jovanovic L, Knopp RH, Brown Z, Conley MR, Park E, Mills JL, Metzger BE, Aarons JH, Holmes LB, Simpson JL, Nalt Inst Child Hlth Human Dev Dia (2001) Declining insulin requirement in the late first trimester of diabetic pregnancy. Diab Care 24:1130–1136
Kasemsri S, Bernardis L, Chlouverakis C, Schnatz JD (1972) Incorporation of 14C-glycerol into adipose tissue lipids of weanling rats with hypothalamic obesity. Proc Soc Exp Biol Med 141:38–42
Knopp RH, Herrera E, Freinkel N (1970) Carbohydrate metabolism in pregnancy.VIII. Metabolism of adipose tissue isolated from fed and fasted pregnant rats during late gestation. J Clin Invest 49:1438–1446
Knopp RH, Sandek CD, Arky RA, O'Sullivan JB (1973) Two phases of adipose tissue metbolism in pregnancy: maternal adaptations for fetal growth. Endocrinology 92:984–988
Koschinsky Kh, Gries FA, Herberg L (1971) Regulation of glycerol kinase by insulin in isolated fat cells and liver of Bar Harbor obese mice. Diabetologia 7:316–322
Lochaya S, Hamilton JC, Mayer J (1963) Lipase and glycerokinase activities in the adipose tissue of obese-hyperglycemic mice. Nature 197:182–183
Lopez Luna P, Maier I, Herrera E (1991) Carcass and tissue fat content in the pregnant rat. Biol Neonate 60:29–38
Maeda N, Funahashi T, Shimomura I (2008) Metabolic impact of adipose tissue and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat Clin Pract Endocrinol Metab 4:627–634
Martin RJ, Lamprey PM (1975) Early development of adipose cell lipogenesis and glycerol utilization in Zucker Obese rats. Proc Soc Exp Biol Med 149:35–39
Mills JL, Jovanovic L, Knopp R, Aarons J, Conley M, Park E, Lee YJ, Holmes L, Simpson JL, Metzger B (1998) Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: the diabetes in early pregnancy study. Metabolism 47:1140–1144
Muñoz C, López-Luna P, Herrera E (1995) Glucose and insulin tolerance tests in the rat on different days of gestation. Biol Neonate 68:282–291
Nye CK, Hanson RW, Kalhan SC (2008) Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J Biol Chem 283:27565–27574
Palacín M, Lasunción MA, Asunción M, Herrera E (1991) Circulating metabolite utilization by periuterine adipose tissue in situ in the pregnant rat. Metabolism 40:534–539
Persico PA, Cerchio GM, Jeffay H (1975) Glycerokinase in mammalian adipose tissue: stimulation by lipogenic substances. Am J Physiol 228:1868–1874
Ramos MP, Crespo-Solans MD, del Campo S, Herrera E (2003) Fat accumulation in the rat during early pregnancy is modulated by enhanced insulin responsiveness. Am J Physiol Endocrinol Metab 285:E318–E328
Ramos P, Herrera E (1995) Reversion of insulin resistance in the rat during late pregnancy by 72-h glucose infusion. Am J Physiol Endocrinol Metab 269:E858–E863
Robinson J, Newsholme EA (1967) Glycerol kinase activities in rat heart and adipose tissue. Biochem J 104:2c–4c
Rodbell M (1964) Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 239:375–380
Ryan EA, O'Sullivan MJ, Skyler JS (1985) Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes 34:380–389
Seibel MJ, Llobera M, Herrera E (1978) Effects of glucose, insulin and adrenalin on glycerol metabolism in adipose tissue from hypothyroid rats. Mol Cell Endocrinol 10:307–318
Sorenson RL, Brelje TC (1997) Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 29:301–307
Tan GD, Debard C, Tiraby C, Humphreys SM, Frayn KN, Langin D, Vidal H, Karpe F (2003) A "futile cycle" induced by thiazolidinediones in human adipose tissue? Nat Med 9:811–812
Taylor WM, Goldrick RB, Ishikawa T (1979) Glycerokinase in rat and human adipose tissue: response to hormonal and dietary stimuli. Horm Metabol Res 11:280–284
Thenen SW, Mayer J (1975) Adipose tissue glycerokinase activity in genetic and acquired obesity in rats and mice. Proc Soc Exp Biol Med 148:953–957
Thenen SW, Mayer J (1975) Hyperinsulinemia and fat cell glycerokinase activity in obese (ob/ob) and diabetic (db/db) mice. Horm Metab Res 8:80–81
Tordjman J, Chauvet G, Quette J, Beale EG, Forest C, Antoine B (2003) Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. J Biol Chem 278:18785–18790
Tordjman J, Khazen W, Antoine B, Chauvet G, Quette J, Fouque F, Beale EG, Benelli C, Forest C (2003) Regulation of glyceroneogenesis and phosphoenolpyruvate carboxykinase by fatty acids, retinoic acids and thiazolidinediones: potential relevance to type 2 diabetes. Biochimie 85:1213–1218
Van Harmelen V, Reynisdottir S, Cianflone K, Degerman E, Hoffstedt J, Nilsell K, Sniderman A, Arner P (1999) Mechanisms involved in the regulation of free fatty acid release from isolated human fat cells by acylation-stimulating protein and insulin. J Biol Chem 274:18243–18251
Villar J, Cogswell M, Kestler E, Castillo P, Menendez R, Repke JT (1992) Effect of fat and fat-free mass deposition during pregnancy on birth weight. Am J Obstet Gynecol 167:1344–1352
Wieland O, Suyter M (1957) Glycerokinase: Isolierung und Eigenschaften des Enzyms. BiochemZ 329:320–331
Acknowledgments
This study was supported by grants from the Ministry of Science and Innovative Technology of Spain (SAF2007-64881 and SAF2008-04518) and from University CEU San Pablo (USP PC 12-09). We thank Brian Crilly for revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrera, E., del Campo, S., Marciniak, J. et al. Enhanced utilization of glycerol for glyceride synthesis in isolated adipocytes from early pregnant rats. J Physiol Biochem 66, 245–253 (2010). https://doi.org/10.1007/s13105-010-0031-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-010-0031-9