Abstract
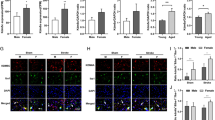
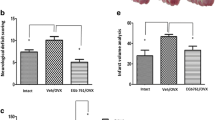
Post-menopausal women become vulnerable to stroke and have poorer outcomes and higher mortality than age-matched men, and previous studies suggested that sex chromosomes play a vital role in mediating stroke sensitivity in the aged. It is unknown if this is due to effects of the X or Y chromosome. The present study used the XY* mouse model (with four genotypes: XX and XO gonadal females and XY and XXY gonadal males) to compare the effect of the X vs. Y chromosome compliment in stroke. Aged (18–20 months) and gonadectomized young (8–12 weeks) mice were subjected to a 60-min middle cerebral artery occlusion. Infarct volume and behavioral deficits were quantified 3 days after stroke. Microglial activation and infiltration of peripheral leukocytes in the aged ischemic brain were assessed by flow cytometry. Plasma inflammatory cytokine levels by ELISA, and brain expression of two X chromosome–linked genes, KDM6A and KDM5C by immunochemistry, were also examined. Both aged and young XX and XXY mice had worse stroke outcomes compared to XO and XY mice, respectively; however, the difference between XX vs. XXY and XO vs. XY aged mice was minimal. Mice with two copies of the X chromosome showed more robust microglial activation, higher brain-infiltrating leukocytes, elevated plasma cytokine levels, and enhanced co-localization of KDM6A and KDM5C with Iba1+ cells after stroke than mice with one X chromosome. The number of X chromosomes mediates stroke sensitivity in aged mice, which might be processed through the X chromosome–linked genes and the inflammatory responses.






Similar content being viewed by others
Abbreviations
- ACA:
-
Anterior cerebral artery
- CNS:
-
Central nervous system
- CV:
-
Crystal violet
- DiI:
-
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- ELISA:
-
Enzyme-linked immunosorbent assay
- FC:
-
Flow cytometry
- FCG:
-
Four core genotypes
- FISH:
-
Fluorescence in situ hybridization
- IHC:
-
Immunohistochemistry
- IL-1β:
-
Interleukin-beta
- iNOS:
-
Inducible NO synthase
- KDM5C:
-
Lysine demethylase 5C
- KDM6A:
-
Lysine-specific demethylase 6A
- MCA:
-
Middle cerebral artery
- MCAO:
-
Middle cerebral artery occlusion
- NDS:
-
Neurological deficit scores
- PCA:
-
Posterior cerebral artery
- PFA:
-
Paraformaldehyde
- TNFα:
-
Tumor necrosis factor alpha
- XCI:
-
X chromosome inactivation
References
Sealy-Jefferson S, et al. Age- and ethnic-specific sex differences in stroke risk. Gend Med. 2012;9(2):121–8.
Petrea Rodica E, et al. Gender differences in stroke incidence and poststroke disability in the Framingham Heart Study. Stroke. 2009;40(4):1032–7.
McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14(5):228–35.
Manwani B, et al. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab. 2015;35(2):221–9.
Liu F, et al. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J Neuroendocrinol. 2012;24(2):319–30.
Schreihofer DA, Ma Y. Estrogen receptors and ischemic neuroprotection: who, what, where, and when? Brain Res. 2013;1514:107–22.
Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Transl Stroke Res. 2013;4(4):390–401.
McCullough LD, et al. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging (Albany NY). 2016;8(7):1432–41.
Chang L, et al. Histone H3 lysine 27 demethylase KDM6B aggravates ischemic brain injury through demethylation of IRF4 and Notch2-dependent SOX9 activation. Mol Ther - Nucleic Acids. 2021.
Felling RJ, Song H. Epigenetic mechanisms of neuroplasticity and the implications for stroke recovery. Exp Neurol. 2015;268:37–45.
Hwang JY, Aromolaran KA, Zukin RS. Epigenetic mechanisms in stroke and epilepsy. Neuropsychopharmacology. 2013;38(1):167–82.
Lindgren A. Stroke genetics: a review and update. J Stroke. 2014;16(3):114–23.
Qi S, et al. X chromosome escapee genes are involved in ischemic sexual dimorphism through epigenetic modification of inflammatory signals. J Neuroinflammation. 2021;18(1):70.
Arnold AP, et al. Cell-autonomous sex determination outside of the gonad. Dev Dyn. 2013;242(4):371–9.
Arnold AP, et al. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol. 2017;37(5):746–56.
Burgoyne PS, Arnold AP. A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol Sex Differ. 2016;7(1):68.
Wistuba J, et al. Male 41, XXY* mice as a model for klinefelter syndrome: hyperactivation of leydig cells. Endocrinology. 2010;151(6):2898–910.
Liu F, McCullough LD. The middle cerebral artery occlusion model of transient focal cerebral ischemia. Methods Mol Biol. 2014;1135:81–93.
Li X, et al. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187(1):94–104.
Liu F, Schafer DP, McCullough LD. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179(1):1–8.
Al Mamun A, et al. Interferon regulatory factor 4/5 signaling impacts on microglial activation after ischemic stroke in mice. Eur J Neurosci. 2018;47(2):140–9.
Al Mamun A, et al. Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc Natl Acad Sci U S A, 2019.
Deacon RMJ, Rawlins JNP. T-maze alternation in the rodent. Nat Protoc. 2006;1(1):7–12.
Swonger AK, Rech RH. Serotonergic and cholinergic involvement in habituation of activity and spontaneous alternation of rats in a Y maze. J Comp Physiol Psychol. 1972;81(3):509–22.
Hsiao KK, et al. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron. 1995;15(5):1203–18.
Wahl F, et al. Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke. 1992;23(2):267–72.
Mirza MA, et al. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J Neuroinflammation. 2015;12:32.
Denker SP, et al. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J Neurochem. 2007;100(4):893–904.
Ritzel RM, et al. Multiparity improves outcomes after cerebral ischemia in female mice despite features of increased metabovascular risk. Proc Natl Acad Sci U S A. 2017;114(28):E5673-e5682.
Dienel A, et al. Microthrombi correlates with infarction and delayed neurological deficits after subarachnoid hemorrhage in mice. Stroke. 2020;51(7):2249–54.
Maeda K, Hata R, Hossmann K-A. Differences in the cerebrovascular anatomy of C57Black/6 and SV129 mice. NeuroReport. 1998;9(7):1317–9.
Goel N, Bale TL. Organizational and activational effects of testosterone on masculinization of female physiological and behavioral stress responses. Endocrinology. 2008;149(12):6399–405.
Chen Y, et al. Targeting microglial activation in stroke therapy: pharmacological tools and gender effects. Curr Med Chem. 2014;21(19):2146–55.
Patel AR, et al. Microglia and ischemic stroke: a double-edged sword. Int J Physiol Pathophysiol Pharmacol. 2013;5(2):73–90.
Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19(4):469–98.
Arnold AP. Y chromosome’s roles in sex differences in disease. Proc Natl Acad Sci U S A. 2017;114(15):3787–9.
Forsberg LA, et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat Genet. 2014;46(6):624–8.
Khan, S.I., et al., Y chromosome, hypertension and cardiovascular disease: is inflammation the answer? Int J Mol Sci, 2019. 20(12).
Teuscher C, et al. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc Natl Acad Sci U S A. 2006;103(21):8024–9.
Sun SL, et al. Y chromosome-linked B and NK cell deficiency in mice. J Immunol. 2013;190(12):6209–20.
Faustino JV, et al. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31(36):12992–3001.
Benakis C, et al. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front Cell Neurosci. 2014;8:461.
Fumagalli S, et al. The ischemic environment drives microglia and macrophage function. Front Neurol. 2015;6:81.
Girard S, et al. Microglia and macrophages differentially modulate cell death after brain injury caused by oxygen-glucose deprivation in organotypic brain slices. Glia. 2013;61(5):813–24.
Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J Clin Investig. 2020;130(6):2777–88.
Jayaraj RL, et al. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16(1):142.
Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082–90.
Bodhankar S, et al. Role for microglia in sex differences after ischemic stroke: importance of M2. Metab Brain Dis. 2015;30(6):1515–29.
Dotson AL, Offner H. Sex differences in the immune response to experimental stroke: implications for translational research. J Neurosci Res. 2017;95(1–2):437–46.
Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Womens Health (Lond). 2011;7(3):319–39.
Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38.
Brockdorff N, Turner BM. Dosage compensation in mammals. Cold Spring Harb Perspect Biol. 2015;7(3):a019406.
Gu L, Walters JR. Evolution of sex chromosome dosage compensation in animals: a beautiful theory, undermined by facts and bedeviled by details. Genome Biol Evol. 2017;9(9):2461–76.
Berletch JB, et al. Genes that escape from X inactivation. Hum Genet. 2011;130(2):237–45.
Iwase S, et al. A mouse model of X-linked intellectual disability associated with impaired removal of histone methylation. Cell Rep. 2016;14(5):1000–9.
Cabrera Zapata, L.E., et al., X-linked histone H3K27 demethylase Kdm6a regulates sexually dimorphic differentiation of hypothalamic neurons. Cell Mol Life Sci, 2021.
Liang G, et al. Distinct localization of histone H3 acetylation and H3–K4 methylation to the transcription start sites in the human genome. Proc Natl Acad Sci USA. 2004;101(19):7357–62.
Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–37.
Lassen S, et al. Ischemia reperfusion induces IFN regulatory factor 4 in renal dendritic cells, which suppresses postischemic inflammation and prevents acute renal failure. J Immunol. 2010;185(3):1976–83.
Almuttaqi H, Udalova IA. Advances and challenges in targeting IRF5, a key regulator of inflammation. FEBS J. 2019;286(9):1624–37.
Funding
This work was supported by funding from the National Institutes of Health: NS108779 (Fudong Liu).
Author information
Authors and Affiliations
Contributions
S.Q., L.D.M., A.P.A, and F.L. designed research; S.Q., A.A.M., C.N. and S.R. performed research; T.W. and S.P.M. contributed to new reagents and data analysis; S.Q. analyzed data; S.Q. and F.L. drafted the manuscript; L.D.M and A.P.A edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experimental procedures were performed in accordance with NIH guidelines for the care and use of laboratory animals and were approved by the Institutional Animal care and use committee of the University of Texas Health Science Center.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, S., Ngwa, C., Al Mamun, A. et al. X, but not Y, Chromosomal Complement Contributes to Stroke Sensitivity in Aged Animals. Transl. Stroke Res. 14, 776–789 (2023). https://doi.org/10.1007/s12975-022-01070-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01070-z