Abstract
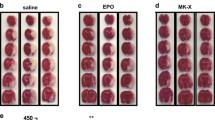
Mammalian cell-produced recombinant human erythropoietin (rhuEPOM) has been shown to be a multimodal neuroprotectant targeting an array of key pathological mechanisms in experimental stroke models. However, the rhuEPOM clinical trials were terminated due to increased risk of thrombosis, largely ascribed to its erythropoietic function. We recently took advantage of a plant-based expression system lacking sialylation capacity to produce asialo-rhuEPOP, a rhuEPO derivative without sialic acid residues. In the present study, we proved that asialo-rhuEPOP is non-erythropoietic by repeated intravenous injection (44 μg/kg bw) in mice showing no increase in hemoglobin levels and red blood cell counts, and confirmed that it is non-immunogenic by measuring humoral response after immunizing the mice. We demonstrate that it is neuroprotective in a cerebral ischemia and reperfusion (I/R) mouse model, exhibiting ~ 50% reduction in cerebral infarct volume and edema, and significant improvement in neurological deficits and histopathological outcome. Our studies further revealed that asialo-rhuEPOP, like rhuEPOM, displays pleiotropic neuroprotective effects, including restoring I/R-interrupted mitochondrial fission and fusion proteins, preventing I/R injury-induced increase in mitophagy and autophagy markers, and inhibiting apoptosis to benefit nerve cell survival. Most importantly, asialo-rhuEPOP lacking erythropoietic activity and immunogenicity holds great translational potential as a multimodal neuroprotectant for stroke treatment.








Similar content being viewed by others
Data Availability
All data associated with this study are present in the paper, which have been confirmed by authors to comply with field standards.
Code Availability
Software application and custom code used to support the published claims have been confirmed by authors to comply with field standards.
References
Barthels D, Das H. Current advances in ischemic stroke research and therapies. BBA – Mol Basis Dis. 2020;1866:165260.
Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17:1391–401.
Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol. 2013;1:185–99.
Muresanu DF, Strilciuc S, Stan A. Current drug treatment of acute ischemic stroke: challenges and opportunities. CNS Drugs. 2019;33:841–7.
Rogalewski A, Schneider A, Ringelstein EB, Schabitz W. Toward a multimodal neuroprotective treatment of stroke. Stroke. 2006;37:1129–36.
Zhang L, Zhang ZG, Chopp M. The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol Sci. 2012;33:415–22.
Liang LJ, Yang JM, Jin XC. Cocktail treatment, a promising strategy to treat acute cerebral ischemic stroke. Med Gas Res. 2016;6:33–8.
Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992;72:449–89.
Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–40.
Patel NS, Nandra KK, Thiemermann C. Bench-to-bedside review: Erythropoietin and its derivatives as therapies in critical care. Crit Care. 2012;16:229.
Souvenir R, Doycheva D, Zhang JH, Tang J. Erythropoietin in stroke therapy: friend or foe. Curr Med Chem. 2015;22:1205–13.
Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–94.
Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264:405–32.
Solling C. Organ-protective and immunomodulatory effects of erythropoietin - an update on recent clinical trials. Basic Clin Pharmacol Toxicol. 2011;110:113–21.
Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–56.
Lippi G, Franchini M, Favaloro EJ. Thrombotic complications of erythropoiesis-stimulating agents. Semin Thromb Hemost. 2010;36:537–49.
Brines M, Patel NS, Villa P, Brines C, Mennini T, De Paola M, et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci USA. 2008;105:10925–30.
Pankratova S, Kiryushko D, Sonn K, Soroka V, Kohler LB, Rathje M, et al. Neuroprotective properties of a novel, non-hematopoietic agonist of the erythropoietin receptor. Brain. 2010;133:2281–94.
Hermanson T, Bennett CL, Macdougall IC. Peginesatide for the treatment of anemia due to chronic kidney disease - an unfulfilled promise. Expert Opin Drug Saf. 2016;15:1421–6.
Erbayraktar S, Grasso G, Sfacteria A, Xie Q, Coleman T, Kreilgaard M, et al. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc Natl Acad Sci USA. 2003;100:6741–6.
Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–42.
Okada T, Sawada T, Kubota K. Asialoerythropoietin has strong renoprotective effects against ischemia-reperfusion injury in a murine model. Transplantation. 2007;84:504–10.
Gan Y, Xing J, Jing Z, Stetler A, Zhand F, Luo Y, et al. Mutant erythropoietin without erythropoietic activity is neuroprotective against ischemic brain injury. Stroke. 2012;43:3071–7.
Price CD, Yang Z, Karlnoski R, Kumar D, Chaparro R, Camporesi EM. Effect of continuous infusion of asialoerythropoietin on short-term changes in infarct volume, penumbra apoptosis and behaviour following middle cerebral artery occlusion in rats. Clin Exp Pharmacol Physiol. 2010;37:185–92.
Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and agiogenesis and improves neurological functions in rats. Stroke. 2004;35:1732–7.
Yamashita T, Nonoguchi N, Ikemoto T, Miyatake S, Kuroiwa T. Asialoerythropoietin attenuates neuronal cell death in the hippocampal CA1 region after transient forebrain ischemia in a gerbil model. Neurol Res. 2010;32:957–62.
Ishii T, Asai T, Oyama D, Fukuta T, Yasuda N, Shimizu K, et al. Amelioration of cerebral ischemia-reperfusion injury based on liposomal drug delivery system with asialo-erythropoietin. J Control Release. 2012;160:81–7.
Weise A, Altmann F, Rodriguez-Franco M, Sjoberg ER, Baumer W, Launhardt H, et al. High level expression of secreted complex glycosylated recombinant human erythropoietin in the Physcomitrella Δ-fuc-t Δ-xyl-t mutant. Plant Biotechnol J. 2007;5:389–401.
Jelkmann W. Recombinant EPO production-points the nephrologist should know. Nephrol Dial Transplant. 2007;22:2749–53.
Kittur FS, Bah M, Archer-Hartmann S, Hung C-Y, Azadi P, Ishihara M, et al. Cytoprotective effect of recombinant human erythropoietin produced in transgenic tobacco plants. PLoS ONE. 2013;8:e76468.
Ma JK, Drake PM, Christou P. The production of recombinant pharmaceutical proteins in plants. Nat Rev Genet. 2003;4:794–805.
Arthur E, Kittur FS, Lin Y, Hung C-Y, Sane DC, Xie J. Plant-produced asialo-erythropoietin restores pancreatic beta-cell function by suppressing mammalian sterile-20-like kinase (MST1) and caspase-3 activation. Front Pharmacol. 2017;8:208.
Kittur FS, Lin Y, Arthur E, Hung C-Y, Li PA, Sane DC, et al. Recombinant asialoerythropoetin protects HL-1 cardiomyocytes from injury via suppression of Mst1 activation. Biochem Biophys Rep. 2019;17:157–68.
Andreux PA, Houtkooper RH, Auwerx J. Pharmacological approaches to restore mitochondrial function. Nat Rev Drug Discov. 2013;12:465–83.
Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38.
Chargelegue D, Vine ND, van Dalleweerd CD, Drake PMW, Ma J. A murine monoclonal antibody produced in transgenic plants with plant-specific glycans is not immunogenic in mice. Transgenic Res. 2000;9:187–94.
Haines BA, Mehta SL, Pratt SM, Warden CH, Li PA. Deletion of mitochondrial uncoupling protein-2 increases ischemic brain damage after transient focal ischemia by altering gene expression patterns and enhancing inflammatory cytokines. J Cereb Blood Flow Metab. 2010;30:1825–33.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–9.
Mehta SL, Kumari S, Mendelev N, Li PA. Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci. 2012;13:79.
Wright DG, Wright EC, Narva AS, Noguchi CT, Rogers PW. Association of erythropoietin dose and route of administration with clinical outcomes for patients on hemodialysis in the Unites States. Clin J Am Soc Nephro. 2015;10:1822–30.
Jelkmann W. Efficacy of recombinant erythropietins: is there unity of international standards? Nephrol Dial Transplant. 2009;24:1366–8.
Simon F, Floros N, Ibing W, Schelzig H, Knapsis A. Neurotherapeutic potential of erythropoietin after ischemic injury of the central nervous system. Neural Regen Res. 2019;14:1309–12.
Kittur FS, Hung C-Y, Zhu CS, Shajahanm A, Azadi P, Thomas MD, et al. Glycoengineering tobacco plants to stably express recombinant human erythropoietin with different N-glycan profiles. Int J Biol Macromol. 2020;157:158–69.
Jin C, Altmann F, Strasser R, Mach L, Schahs M, Kunert R, et al. A plant-derived human monoclonal antibody induces an anti-carbohydrate immune response in rabbits. Glycobiology. 2008;18:235–41.
Ward BJ, Landry N, Trépanier S, Mercier G, Dargis M, Couture M, et al. Human antibody response to N-glycans present on plant-made influenza virus-like particle (VLP) vaccines. Vaccine. 2014;32:6098–106.
Wang X, Jiang D, Shi J, Yang D. Expression of α-1,6-fucosyltransferase (FUT8) in rice grain and immunogenicity evaluation of plant-specific glycans. J Biotechnol. 2017;20:111–21.
Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, et al. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44:1167–71.
Balog J, Mehta SL, Vemuganti R. Mitochondrial fission and fusion in secondary brain damage after CNS insults. J Cereb Blood Flow Metab. 2016;36:2022–33.
Liu F, Lu J, Manaenko A, Tang J, Hu Q. Mitochondria in ischemic stroke: new insight and implications. Aging Dis. 2018;9:924–37.
Cheung EC, Mcbride HM, Slack RS. Mitochnodrial dynamics in the regulation of neuronal cell death. Apoptosis. 2007;12:979–92.
Otera H, Mihara K. Mitochondrial dynamics: functional link with apoptosis. Intl J Cell Biol. 2012;2012:821676.
Kumar R, Bukowski MJ, Wider JM, Reynolds CA, Calo L, Lepore B, et al. Mitochondrial dynamics following global cerebral ischemia. Mol Cell Neurosci. 2016;76:68–75.
Burman JL, Pickles S, Wang C, Sekine S, Vargas J, Zhang Z, et al. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J Cell Biol. 2017;216:3231–47.
Ma K, Chen G, Li W, Keep O, Zhu Y, Quan C. Mitophagy, mitochondrial homeostasis, and cell fate. Front Cell Dev Biol. 2020;8:467.
Sun Y, Zhu Y, Zhong X, Chen X, Wang J, Ying G. Crosstalk between autophagy and cerebral ischemia. Front Neurosci. 2019;12:1022.
Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–67.
Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol. 2010;221:117–24.
Levine B, Liu R, Dong X, Zhong Q. Beclin orthologs: integrative hubs of cell signaling, membrane trafficking, and physiology. Trends Cell Biol. 2015;25:533–44.
Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501.
Ehrenreich H, Aust C, Krampe H, Jahn H, Jacob S, Hermann M, et al. Erythropoietin: novel approaches to neuroprotection in human brain disease. Metab Brain Dis. 2004;19:195–206.
Bardor M, Fareeuw C, Fitchette AC, Gilbert D, Galas L, Trottein F, et al. Immunoreactivity of two typical plant glycol-epitopes, core α(1,3)-fucose and core xylose. Glycobiology. 2003;13:427–34.
Rup B, Alon S, Amit-Cohen BC, Almon EB, Chertkoff R, Tekoah Y, et al. Immunogenicity of glycans on biotherapeutic drugs produced in plant expression systems-the taliglucerase alfa story. PLoS ONE. 2017;12:e0186211.
Debierre-Grockiego F. Anti-apoptotic role of STAT5 in haematopoietic cells and in the pathogenesis of malignancies. Apoptosis. 2004;9:717–28.
Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1356–62.
Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/protein kinase B inhibits cell death by preventing the release of cyt c from mitochondria. Mol Cell Biol. 1999;19:5800–10.
Szabo A, Sumegi K, Fekete K, Hocsak E, Debreceni B, Setalo G Jr, et al. Activation of mitochondrial fusion provides a new treatment for mitochondria-related diseases. Biochem Pharmacol. 2018;150:86–96.
Ong SB, Hall AR, Dongworth RK, Kalkhoran S, Pyakurel A, Scorrano L, et al. Akt protects the heart against ischaemia-reperfusion injury by modulating mitochondrial morphology. Thromb Haemost. 2015;113:513–21.
Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther. 2012;18:250–60.
Cook SJ, Stuart K, Gilley R, Sale MJ. Control of cell death and mitochondrial fission by ERK1/2 MAP kinase signaling. FEBS J. 2017;284:4177–95.
Philo JS, Aoki KH, Arakawa T, Narhi LO, Wen J. Dimerization of the extracellular domain of the erythropoietin (EPO) receptor by EPO: one high-affinity and one low-affinity interaction. Biochemistry. 1996;35:1681–91.
Darling RJ, Kuchibhotla U, Glaesner W, Micanovic R, Witcher DR, Beals JM. Glycosylation of erythropoietin affects receptor binding kinetics: role of electrostatic interactions. Biochemistry. 2002;41:14524–31.
Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA. 2004;101:14907–12.
Ostrowski D, Heinrich R. Alternative erythropoietin receptors in the nervous system. J Clin Med. 2018;7:24.
Shing KSCT, Broughton SE, Nero TL, Gillinder K, Ilsley MD, Ramshaw H, et al. EPO does not promote interaction between the erythropoietin and beta-common receptors. Sci Rep. 2018;8:12457.
Acknowledgements
We thank Qingping He for the help with immunofluorescence assay.
Funding
This work was supported by the National Institute of General Medical Sciences grant SC1GM111178 to Jiahua Xie.
Author information
Authors and Affiliations
Contributions
F.S.K, D.C.S, P.A.L, and J.X. designed research; M.H, F.S.K, C.-Y.H, and J.Z. performed the experiments; M.H, F.S.K, P.A.L., J.Z. L.J., D.C.S, and J.X. analyzed the data. M.H, F.S.K., and J.X. drafted the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Ethics Approval
This study was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Association and Accreditation of Laboratory Animal Care International guidelines and under the authorization of the Institutional Animal Care and Use Committee at the North Carolina Central University, Durham, NC, USA.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
JX, FK, and C-Y H are inventors of filed patent “Methods for the production of cytoprotective asialo-erythropoietin in plants and its purification from plant tissues” (PCT NUMBER: US2013031382, pending).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, M., Kittur, F.S., Hung, CY. et al. A Novel Plant-Produced Asialo-rhuEPO Protects Brain from Ischemic Damage Without Erythropoietic Action. Transl. Stroke Res. 13, 338–354 (2022). https://doi.org/10.1007/s12975-021-00943-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-021-00943-z