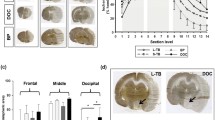
Abstract
Most large vessel stroke patients have permanent occlusion, for which there are no current treatment options. Recent case studies have indicated delayed recanalization, that is recanalization outside of the 6-h treatment window, may lead to improved outcome. We hypothesized that delayed recanalization will restore cerebral blood flow, leading to improved function in rats. Male SD rats were subjected to pMCAO or sham surgery. Delayed recanalization was performed on either day 3, 7, or 14 after pMCAO in a subset of animals. Cerebral blood flow was monitored during suture insertion, during recanalization, and then at sacrifice. Neurological function was evaluated for 1 week after delayed recanalization and at 4 weeks post-ictus. After sacrifice, cerebral morphology was measured. Compared to no treatment, delayed recanalization restored cerebral blood flow, leading to sensorimotor recovery, improved learning and memory, reduced infarct volume, and increased neural stem/progenitor cells within the infarction. The data indicate that earlier delayed recanalization leads to better functional and histological recovery. Yet, even restoring cerebral blood flow 14 days after pMCAO allows for rats to regain sensorimotor function. This exploratory study suggests that delayed recanalization may be a viable option for treatment of permanent large vessel stroke.












Similar content being viewed by others
References
Rai AT. Red pill, blue pill: reflections on the emerging large vessel stroke ‘market’. J Neurointerventional Surg. 2015;7(9):623–5. https://doi.org/10.1136/neurintsurg-2015-011971.
Gonzalez RG, Furie KL, Goldmacher GV, Smith WS, Kamalian S, Payabvash S, et al. Good outcome rate of 35% in IV-tPA-treated patients with computed tomography angiography confirmed severe anterior circulation occlusive stroke. Stroke. 2013;44(11):3109–13. https://doi.org/10.1161/STROKEAHA.113.001938.
Yoshimura S, Sakai N, Okada Y, Kitagawa K, Kimura K, Tanahashi N, et al. Efficacy of endovascular treatment for acute cerebral large-vessel occlusion: analysis of nationwide prospective registry. J Stroke Cerebrovasc Dis. 2014;23(5):1183–90. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.10.014.
Saver JL. Improving reperfusion therapy for acute ischaemic stroke. Thromb Haemost. 2011;9(Suppl 1):333–43.
McBride DW, Zhang JH. Precision stroke animal models: the permanent MCAO model should be the primary model, not transient MCAO. Transl Stroke Res. 2017;8(5):397–404. https://doi.org/10.1007/s12975-017-0554-2.
Terada T, Yamaga H, Tsumoto T, Masuo O, Itakura T. Use of an embolic protection system during endovascular recanalization of a totally occluded cervical internal carotid artery at the chronic stage. Case report J Neurosurg. 2005;102(3):558–64. https://doi.org/10.3171/jns.2005.102.3.0558.
Yu W, Kostanian V, Fisher M. Endovascular recanalization of basilar artery occlusion 80 days after symptom onset. Stroke. 2007;38(4):1387–9. https://doi.org/10.1161/01.STR.0000260186.93667.a2.
Payne S. DAWN Trial Results Demonstrate a 73% Reduction in Disability in Stroke Patients Treated up to 24 Hours. 2017. http://www.prweb.com/releases/2017/05/prweb14339427.htm. Accessed May 22, 2017 2017.
Mu J, Ostrowski RP, Krafft PR, Tang J, Zhang JH. Serum leptin levels decrease after permanent MCAo in the rat and remain unaffected by delayed hyperbaric oxygen therapy. Med Gas Res. 2013;3(1):8. https://doi.org/10.1186/2045-9912-3-8.
Mu J, Ostrowski RP, Soejima Y, Rolland WB, Krafft PR, Tang J, et al. Delayed hyperbaric oxygen therapy induces cell proliferation through stabilization of cAMP responsive element binding protein in the rat model of MCAo-induced ischemic brain injury. Neurobiol Dis. 2013;51:133–43. https://doi.org/10.1016/j.nbd.2012.11.003.
Shanbhag NC, Henning RH, Schilling L. Long-term survival in permanent middle cerebral artery occlusion: a model of malignant stroke in rats. Sci Rep. 2016;6(1) https://doi.org/10.1038/srep28401.
Tulsulkar J, Glueck B, Hinds TD, Shah ZA. Ginkgo Biloba extract prevents female mice from ischemic brain damage and the mechanism is independent of the HO1/Wnt pathway. Transl Stroke Res. 2016;7(2):120–31. https://doi.org/10.1007/s12975-015-0433-7.
McBride DW, Klebe D, Tang J, Zhang JH. Correcting for brain Swelling’s effects on infarct volume calculation after middle cerebral artery occlusion in rats. Transl Stroke Res. 2015;6(4):323–38. https://doi.org/10.1007/s12975-015-0400-3.
Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130(Pt 6):1643–52.
Slivka A, Murphy E, Horrocks L. Cerebral edema after temporary and permanent middle cerebral artery occlusion in the rat. Stroke. 1995;26(6):1061–5; discussion 5-6. https://doi.org/10.1161/01.STR.26.6.1061.
Yao Y, Miao W, Liu Z, Han W, Shi K, Shen Y, et al. Dimethyl fumarate and monomethyl fumarate promote post-ischemic recovery in mice. Transl Stroke Res. 2016;7(6):535–47. https://doi.org/10.1007/s12975-016-0496-0.
Roof RL, Schielke GP, Ren X, Hall ED. A comparison of long-term functional outcome after 2 middle cerebral artery occlusion models in rats. Stroke. 2001;32(11):2648–57. https://doi.org/10.1161/hs1101.097397.
Li P, Murphy TH. Two-photon imaging during prolonged middle cerebral artery occlusion in mice reveals recovery of dendritic structure after reperfusion. J Neurosci. 2008;28(46):11970–9. https://doi.org/10.1523/JNEUROSCI.3724-08.2008.
Trueman RC, Diaz C, Farr TD, Harrison DJ, Fuller A, Tokarczuk PF, et al. Systematic and detailed analysis of behavioural tests in the rat middle cerebral artery occlusion model of stroke: tests for long-term assessment. J Cereb Blood Flow Metab. 2016;37(4):1349–61. https://doi.org/10.1177/0271678X16654921.
Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203(2):555–67. https://doi.org/10.1016/j.expneurol.2006.09.006.
Goldmacher GV, Nasser R, Lee DY, Yigit S, Rosenwasser R, Iacovitti L. Tracking transplanted bone marrow stem cells and their effects in the rat MCAO stroke model. PLoS One. 2013;8(3):e60049. https://doi.org/10.1371/journal.pone.0060049.
Guan W, Kozak A, El-Remessy AB, Johnson MH, Pillai BA, Fagan SC. Acute treatment with candesartan reduces early injury after permanent middle cerebral artery occlusion. Transl Stroke Res. 2011;2(2):179–85. https://doi.org/10.1007/s12975-010-0061-1.
Hopkins LN, Budny JL. Complications of intracranial bypass for vertebrobasilar insufficiency. J Neurosurg. 1989;70(2):207–11. https://doi.org/10.3171/jns.1989.70.2.0207.
Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316(12):1279–1288. https://doi.org/10.1001/jama.2016.13647.
Giffard C, Young AR, Mézenge F, Derlon J-M, Baron J-C. Histopathological effects of delayed reperfusion after middle cerebral artery occlusion in the anesthetized baboon. Brain Res Bull. 2005;67(4):335–40. https://doi.org/10.1016/j.brainresbull.2005.08.001.
Linfante I, Cipolla MJ. Improving reperfusion therapies in the era of mechanical thrombectomy. Transl Stroke Res. 2016;7(4):294–302. https://doi.org/10.1007/s12975-016-0469-3.
Sun N, Keep RF, Hua Y, Xi G. Critical role of the sphingolipid pathway in stroke: a review of current utility and potential therapeutic targets. Transl Stroke Res. 2016;7(5):420–38. https://doi.org/10.1007/s12975-016-0477-3.
Liu H, Wang Y, Xiao Y, Hua Z, Cheng J, Jia J. Hydrogen sulfide attenuates tissue plasminogen activator-induced cerebral hemorrhage following experimental stroke. Transl Stroke Res. 2016;7(3):209–19. https://doi.org/10.1007/s12975-016-0459-5.
Petrone AB, O’Connell GC, Regier MD, Chantler PD, Simpkins JW, Barr TL. The role of arginase 1 in post-stroke immunosuppression and ischemic stroke severity. Transl Stroke Res. 2016;7(2):103–10. https://doi.org/10.1007/s12975-015-0431-9.
Alhadidi Q, Bin Sayeed MS, Shah ZA. Cofilin as a promising therapeutic target for ischemic and hemorrhagic stroke. Transl Stroke Res. 2016;7(1):33–41. https://doi.org/10.1007/s12975-015-0438-2.
Hasegawa Y, Nakagawa T, Uekawa K, Ma M, Lin B, Kusaka H, et al. Therapy with the combination of amlodipine and irbesartan has persistent preventative effects on stroke onset associated with BDNF preservation on cerebral vessels in hypertensive rats. Transl Stroke Res. 2016;7(1):79–87. https://doi.org/10.1007/s12975-014-0383-5.
Shi Y, Leak RK, Keep RF, Chen J. Translational stroke research on blood-brain barrier damage: challenges, perspectives, and goals. Transl Stroke Res. 2016;7(2):89–92. https://doi.org/10.1007/s12975-016-0447-9.
Gueniau C, Oberlander C. The kappa opioid agonist niravoline decreases brain edema in the mouse middle cerebral artery occlusion model of stroke. J Pharmacol Exp Therapeutics. 1997;282(1):1–6.
Henninger N, Fisher M. Extending the time window for endovascular and pharmacological reperfusion. Transl Stroke Res. 2016;7(4):284–93. https://doi.org/10.1007/s12975-015-0444-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 4858 kb)
Rights and permissions
About this article
Cite this article
McBride, D.W., Wu, G., Nowrangi, D. et al. Delayed Recanalization Promotes Functional Recovery in Rats Following Permanent Middle Cerebral Artery Occlusion. Transl. Stroke Res. 9, 185–198 (2018). https://doi.org/10.1007/s12975-018-0610-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-018-0610-6