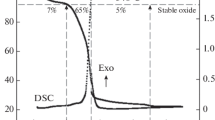
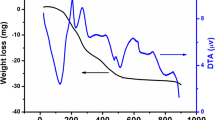
Abstract
Nanocrystalline La0.5Sr0.5MnO3 (LSMO) perovskite-sized powders were successfully synthesized at heating low temperature and time using the Pechini method based on polyesterification between citric acid and ethylene glycol. The electroactivity of carbon-supported perovskite (LSMO/C) for the oxygen reduction reaction (ORR) was evaluated. The results showed that the electroactivity of LSMO/C largely depends on the calcination temperature, type of electrolyte, and mass loading. It increased remarkably with the calcination temperature in 0.1 M KOH. The ORR performance was examined in KOH, NaOH, and K2SO4. Higher electroactivity was recorded in KOH electrolyte, as compared to NaOH and K2SO4.

Single-phase nanocrystalline La0.5Sr0.5MnO3 (LSMO) perovskite oxides were obtained via the Pechini method at 600 °C. The carbon-supported perovskite (LSMO/C) (heat treated at 700 °C) showed a pronounced oxygen reduction activity as compared to those heat treated at 500 and 600 °C.










Similar content being viewed by others
References
K. Gong, F. Du, Z. Xia, M. Durstock, L. Dai, Science 323, 760 (2009)
E.M. Garcia, H.A. Tarôco, T. Matencio, R.Z. Domingues, J.A. dos Santos, Int. J. Hydrog. Energy 37, 6400 (2012)
J. Suntivich, H.A. Gasteiger, N. Yabuuchi, H. Nakanishi, J.B. Goodenough, Y. Shao-Horn, Nat. Chem. 3, 546 (2011)
D. Geng, Y. Chen, Y. Chen, Y. Li, R. Li, X. Sun, S. Ye, S. Knights, Energy Environ. Sci. 4, 760 (2011)
C. Jin, X. Cao, L. Zhang, C. Zhang, R. Yang, J. Power Sources 241, 225 (2013)
C. Zhu, A. Nobuta, I. Nakatsugawa, T. Akiyama, Int. J. Hydrog. Energy 38, 13238 (2013)
J. Tulloch, S.W. Donne, J. Power Sources 188, 359 (2009)
E. Siebert, A. Hammouche, M. Kleitz, Electrochim. Acta 40, 1741 (1995)
P.A. Lessing, Am. Ceram. Soc. Bull. 68, 1002 (1989)
W. Liu, G.C. Farrington, F. Chaput, B. Dunn, J. Electrochem. Soc. 143, 879 (1996)
S.C. Zhang, G.L. Messing, W. Huebner, M.M. Coleman, J. Mater. Res. 5, 1806 (1990)
M. Pechini, US Patent 3.330.697 (1967)
D. Thiele, A. Züttel, J. Power Sources 183, 590 (2008)
T. Poux, F.S. Napolskiy, T. Dintzer, G. Kéranguéven, S.Y. Istomin, G.A. Tsirlina, E.V. Antipov, E.R. Savinova, Catal. Today 189, 83 (2012)
M.S.G. Baythoun, F.R. Sale, J. Mater. Sci. 17, 2757 (1982)
B.D. Cullity, Answers to Problems: Elements of X-Ray Diffraction (Wesley Publishing Company, Addison, 1978)
W.S. Kim, G. Anoop, H.J. Lee, S.S. Lee, J.H. Kwak, H.J. Lee, J.Y. Jo, J. Catal. 344, 578 (2016)
S.K. Tiwari, P. Chartier, R.N. Singh, J. Electrochem. Soc. 142, 148 (1995)
S. Levine, A.L. Smith, Discuss. Faraday Soc. 52, 290 (1971)
G. Kéranguéven, S. Royer, E. Savinova, Electrochem. Commun. 50, 28 (2015)
M. Bursell, M. Pirjamali, Y. Kiros, Electrochim. Acta 47, 1651 (2002)
D. Strmcnik, D.F. van der Vliet, K.-C. Chang, V. Komanicky, K. Kodama, H. You, V.R. Stamenkovic, N.M. Marković, J. Phys. Chem. Lett. 2, 2733 (2011)
W. Jin, H. Du, S. Zheng, H. Xu, Y. Zhang, J. Phys. Chem. B 114, 6542 (2010)
J.C. Li, P. Chang, J. Chem. Phys. 23, 518 (1955)
A.J. Bard, L.R. Faulkner, J. Leddy, C.G. Zoski, Electrochemical Methods: Fundamentals and Applications (Wiley, New York, 1980)
M. Görgényi, J. Dewulf, H. Van Langenhove, K. Héberger, Chemosphere 65, 802 (2006)
J. Suntivich, E.E. Perry, H.A. Gasteiger, Y. Shao-Horn, Electrocatalysis 4, 49 (2013)
D. Strmcnik, K. Kodama, D. van der Vliet, J. Greeley, V.R. Stamenkovic, N.M. Marković, Nat. Chem. 1, 466 (2009)
K.L. Hsueh, E.R. Gonzalez, S. Srinivasan, Electrochim. Acta 28, 691 (1983)
K.F. Blurton, E. McMullin, Energy Convers. 9, 141 (1969)
C. Domínguez, F.J. Pérez-Alonso, J.L. Gómez de la Fuente, S.A. Al-Thabaiti, S.N. Basahel, A.O. Alyoubi, A.A. Alshehri, M.A. Peña, S. Rojas, J. Power Sources 271, 87 (2014)
S. Taylor, E. Fabbri, P. Levecque, T.J. Schmidt, O. Conrad, Electrocatalysis 7, 287 (2016)
L. Timperman, Y.J. Feng, W. Vogel, N. Alonso-Vante, Electrochim. Acta 55, 7558 (2010)
Y. Feng, T. He, N. Alonso-Vante, Electrochim. Acta 54, 5252 (2009)
Y. Feng, T. He, N. Alonso-Vante, Chem. Mater. 20, 26 (2008)
N. Alonso-Vante, ChemPhysChem 11, 2732 (2010)
T. Poux, A. Bonnefont, A. Ryabova, G. Kerangueven, G.A. Tsirlina, E.R. Savinova, Phys. Chem. Chem. Phys. 16, 13595 (2014)
L. Demarconnay, C. Coutanceau, J.-M. Léger, Electrochim. Acta 49, 4513 (2004)
E. Yeager, J. Mol. Catal. 38, 5 (1986)
Acknowledgements
This research was financially supported by the “Accord-programme algéro-français: Projet Tassili N°14MDU911.” The support is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khellaf, N., Kahoul, A., Naamoune, F. et al. Electrochemistry of Nanocrystalline La0.5Sr0.5MnO3 Perovskite for the Oxygen Reduction Reaction in Alkaline Medium. Electrocatalysis 8, 450–458 (2017). https://doi.org/10.1007/s12678-017-0397-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-017-0397-3