Abstract
Endometrial stromal sarcomas are rare tumors that recur long after initial excision. We report a case of recurrent endometrial stromal sarcoma treated with the aromatase inhibitor letrozole and an overview of research completed to date. A 59-year-old female presented with new abdominal onset pain, fatigue, and nausea. She had a computed tomography that showed a pelvic mass, an enlarged right internal iliac lymph node, an atypical liver hemangioma, and a severe left hydronephrosis. The patient underwent an exploratory laparotomy, and attempted surgical resection of the pelvic mass was attempted. Pathology was consistent with recurrent endometrial stromal sarcoma. Since these tumors are hormonally sensitive, the patient was started on letrozole 2.5 mg daily. The patient had complete clinical and radiographic response by 11 months. After 24 months of therapy, the patient remained free of disease. Endometrial stromal sarcomas are hormonally sensitive tumors. Progestins function to decrease the effects of estrogen on target cells and have been used for primary therapy of endometrial stromal sarcomas. Aromatase inhibitors block peripheral synthesis of estrogen. Letrozole is a type 2 aromatase inhibitor that effectively reduces serum estrogen levels. Letrozole has been described as treatment for endometrial stromal sarcoma. Letrozole is well tolerated and is a good option for long-term management of this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uterine sarcomas are rare and comprise 2% of all uterine malignancies, with an incidence of 1.7 per million in the United States [1, 2]. Endometrial stromal sarcomas are the least common type and represent only 0.2% of all uterine cancers [3]. Endometrial stromal sarcomas are generally indolent tumors, characterized by recurrences long after initial excision. We report on a long-term survivor of an endometrial stromal sarcoma now treated with letrozole, an aromatase inhibitor.
Case Report and Results
A 59-year-old female presented with complaints of fatigue and constipation. Her history was significant for endometrial stromal sarcoma, which was initially treated in 1987 with a total abdominal hysterectomy, bilateral salpingoophorectomy, and resection of the pelvic mass. The disease involved the right pelvic side wall and required ureterectomy and reimplantation of the ureter. The patient was maintained postoperatively on long-term oral megestrol acetate. The patient took megestrol acetate for 15 years. The patient had an abdominal recurrence in 2002 on megestrol acetate and underwent complete resection of the tumor. Megestrol acetate was stopped.
In 2008, she presented with new onset abdominal pain, fatigue, and nausea. Computerized tomography (CT) scan showed a 5-cm pelvic mass posterior and lateral to the bladder, a 1.5-cm right internal iliac lymph node, and a 2-cm liver lesion. The liver lesion was noted to be an atypical hemangioma. The mass caused severe left hydronephrosis. Exploratory laparotomy was performed with the intention of optimal tumor debulking. A left ureteral stent was placed only to the midureter. Intra-abdominal disease on the small bowel was a confirmed recurrence by frozen section. The large mass was also resected but extensive pelvic side wall disease was deemed unresectable. Final pathology was consistent with recurrent endometrial stromal sarcoma and showed multiple nodules comprised of cytologically bland small spindle cells arranged in a fascicular and storiform pattern. It was also noted that there were cells with rare mitoses, and there were no atypical mitotic figures. The tumor was positive for CD10, and estrogen and progesterone receptors. In addition, the tumor was negative for pan-cytokeratin, S100 protein, and CD117. The tumor was considered to be recurrent endometrial stromal sarcoma. Postoperatively, a left percutaneous nephroureteral catheter was placed by interventional radiology.
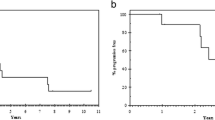
The patient was placed on letrozole 2.5 mg daily. Three months after starting therapy, a CT scan showed partial response in pelvic disease, and complete radiographic and clinical response was documented at 11 months. The liver hemangioma was stable on repeat imaging. The patient has been continued on letrozole and remains disease free at 24 months.
Conclusions
Endometrial stromal sarcomas are rare indolent tumors. Historically, endometrial stromal sarcomas were subdivided into high-grade and low-grade tumors. However, it is now recognized that previously designated high-grade tumors represent a separate disease process with a prognosis that differs from that of low-grade endometrial sarcomas [4, 5]. High-grade tumors are now designated undifferentiated endometrial sarcoma and are described as necrotic tumors with marked atypia and high mitotic activity. Since high mitotic activity has not been shown to correlate to poor prognosis, mitotic activity is no longer used to differentiate high-grade tumors from low-grade tumors [5]. In addition, undifferentiated stromal sarcomas commonly do not express hormonal receptors [5].
Endometrial stromal sarcomas are hormonally sensitive tumors that are known to express estrogen and progesterone receptors [6]. Endometrial stromal sarcomas are vascular, infiltrating tumors that extend into the lymphovascular space and histologically resemble secretory endometrial stroma [4]. Recurrent disease has been reported in patients treated with estrogen replacement therapy and tamoxifen [7, 8]. In a retrospective study of 11 women with endometrial stromal sarcoma, Pink et al. noted progression of disease in three out of 10 patients treated with tamoxifen, and metastases in an additional five out of 10 treated with estrogen replacement therapy [7]. Of those treated with estrogen replacement therapy, cessation of estrogen therapy resulted in no further disease progression [7].
In a review of 10 cases of metastatic endometrial stromal sarcoma, it was noted that eight of the 10 cases expressed estrogen receptor (ER) alpha, and none of the cases expressed ER beta (ERβ) [8]. It has been speculated that the loss of ERβ expression may be a marker of malignant potential [8]. The expression of estrogen and progesterone receptors explains the response of these tumors to hormonal therapy [6]. Progestins downregulate the estrogen receptor, inhibit estrogen-mediated growth factors and affect estrogen metabolism and clearance, thereby decreasing the effects of estrogen on target cells [9]. Progestin therapy has historically been considered the primary treatment of endometrial stromal sarcoma. Adverse effects of progestin therapy include weight gain, depression, and thromboembolic events [9].
A trial using the progesterone receptor modulator mifepristone as hormonal therapy was recently completed [10]. Mifepristone binds to progesterone and glucocorticoid receptors, resulting in an antagonistic effect [11]. However, in the absence of progesterone, mifepristone can result in the activation of genes typically activated by progesterone, thereby exhibiting agonistic properties [12]. Mifepristone has also been noted to have androgenic activity [12]. Ramondetta et al. noted that, of the 12 patients treated with mifepristone, three achieved stable disease [10]. Stable disease was achieved in one of the two patients with endometrial stromal sarcoma enrolled in the study. Although the use of mifepristone resulted in a 25% stable disease response, the authors did note that combining mifepristone with other agents may improve the response rate and deserves further study [10]. While the authors noted that mifepristone was well tolerated, adverse effects include nausea, vomiting, weight loss, hot flushes, and hair loss [10, 12].
Aromatase inhibitors are used in the treatment of breast cancer and proposed as a potential treatment of endometrial stromal sarcoma. Aromatase expression has been observed in endometrial stromal sarcomas [13]. Aromatase inhibitors block the synthesis of estrogen peripherally and within the tumor [14]. Aminoglutethimide, a first generation aromatase inhibitor, has been used in the treatment of metastatic endometrial stromal sarcoma [15]. However, it is a nonspecific nonsteroidal aromatase inhibitor that requires the concomitant administration of corticosteroids due to its effect on cholesterol metabolism [16]. Letrozole is an oral type 2 aromatase inhibitor that works by reversibly interacting with the cytochrome P450 portion of the aromatase complex [16, 17]. In vivo, letrozole at a dose of 2.5 mg/day has been shown to inhibit aromatase activity by greater than 98% [18]. Letrozole has been shown to effectively decrease estrogen levels. In one study, a 95% reduction in the levels of serum estradiol, estrone, estrone sulfate, and urinary estrogen was noted after 2 weeks of treatment of 42 postmenopausal breast cancer patients with letrozole 0.1 to 5 mg/day [17]. Therefore, letrozole would theoretically be more effective than progestins at achieving hormonal control of estrogen and progesterone receptor-positive endometrial stromal sarcoma.
Endometrial stromal sarcoma is a rare disease, and therefore, no prospective studies of therapy with aromatase inhibitors have been completed. Various case reports and case series have shown response of recurrences of endometrial stromal sarcoma to treatment with letrozole [19–21]. Two case reports detail patients who have good responses to long-term therapy with letrozole used as primary hormonal treatment after surgical resection. Leunen et al. noted a case of a patient with recurrent low-grade stromal sarcoma who had stable disease at 36 months after treatment with letrozole, whereas Kraus noted a patient that was disease free after 39 months of treatment [19, 20]. Maluf et al. noted that one patient who had a recurrence after radiotherapy responded to letrozole therapy for 9 months prior to having another recurrence [21].
Letrozole is a good option for long-term management of recurrences. Further studies are needed to evaluate the efficacy and duration of such treatment on a larger scale. Theoretically, therapy with letrozole can be continued as either as long as there is stable disease or no recurrences. Letrozole is administered orally and well tolerated, and the associated adverse effects are considered to be mild [22]. The major adverse effects include hot flashes, vaginal dryness, myalgia, and fatigue [22].
Long-term use of aromatase inhibitors is also associated with increased risk of osteoporosis [22]. The effect of aromatase inhibitors on bone density has been studied in breast cancer patients, and a higher rate of bone density loss, osteoporosis, and fracture has been noted in patients treated with aromatase inhibitors [23]. Breast cancer patients on aromatase inhibitors have a 2.6% annual rate of bone density loss, whereas the annual rate in postmenopausal women is 1% [23]. Therefore, any patient on long-term letrozole therapy should be screened for osteoporosis. The National Osteoporosis Foundation 2008 recommendations note that aromatase inhibitor therapy can either promote or play a role in the development of osteoporosis [24]. Therefore, bone mineral density testing is indicated in any patient currently on therapy with aromatase inhibitors. Testing should be repeated no more frequently than every 2 years unless the patient has developed new risk factors [25]. All patients should be evaluated for risk factors for falls [24]. In order to reduce risk of fracture, all patients should receive counseling on increasing calcium and vitamin D intake and limiting their use of alcohol and tobacco and weight-bearing exercise [24].
References
Boutselis JG, Ullery JC (1962) Sarcoma of the uterus. Obstet Gynecol 20(1):23–35
Harlow BW, Weiss NS, Lofton S (1986) The epidemiology of sarcomas of the uterus. J Natl Cancer Inst 76:399–402
Koss LG, Spiro RH, Brunschwig A (1965) Endometrial stromal sarcoma. Surg Gynecol Obstet 121:531–537
Moinfar F, Masood A, Tavassoli FA (2007) Uterine sarcomas. Path 39(1):55–71
Brown L (2008) Pathology of uterine malignancies. Clin Oncol 20:433–447
Katz L et al (1987) Endometrial stromal sarcoma: a clinicopathologic study of 11 cases with determination of estrogen and progestin receptor levels in three tumors. Gynecol Oncol 26:87–97
Pink D et al (2006) Harm or benefit of hormonal treatment in metastatic low-grade endometrial stromal sarcoma: single center experience with 10 cases and review of the literature. Gynecol Oncol 101:464–469
Chu MC et al (2003) Low-grade stromal sarcoma: hormonal aspects. Gynecol Oncol 90:170–176
Reich O, Regauer S (2007) Hormonal therapy of endometrial stromal sarcoma. Cur Opin in Oncol 19:347–352
Ramondetta L et al (2009) Phase 2 trial of mifepristone (RU-486) in advanced or recurrent endometrioid adenocarcinoma or low-grade stromal sarcoma. Cancer 115:1867–1874
Van Look P, von Hertzen H (1995) Clinical uses of antiprogestogens. Hum Repro Update 1(1):19–34
Spitz IM, Bardin CW (2003) Mifepristone (RU 486)—a modulator of progestin and glucocorticoid action. New Engl J Med 329:404–412
Reich O, Regauer S (2004) Aromatase expression in low-grade stromal sarcomas: an immunohistochemical study. Mod Path 17:104–108
Reich O, Regauer S (2005) Hormonal therapy with aromatase inhibitors for patients with endometrial stromal sarcoma. Letter Gynecol Oncol 98(1):173–174
Spano JP et al (2003) Long-term survival of patients given hormonal therapy for metastatic endometrial stromal sarcoma. Med Oncol 20(1):87–93
Gadduci A, Cosio S, Genazzani AR (2004) Use of estrogen antagonist and aromatase inhibitors in breast cancers and hormonally sensitive tumors of the uterine body. Curr Opin Investig Drugs 5(10):1031–1044
Lamb HM, Adkins JC (1998) Letrozole: a review of its use in postmenopausal women with advanced breast cancer. Drugs 56(6):1125–1140
Dowsett M et al (1995) In vivo measurement of aromatase inhibition by letrozole (CGS 20267) in postmenopausal patients with breast cancer. Clin Cancer Res 1:1511–1515
Krauss K et al (2007) Management of late recurrence of a low-grade endometrial stromal sarcoma (LGESS): treatment with letrozole. Anticancer Res 27:3477–3480
Leunen M et al (2004) Low grade stromal sarcoma treated with the aromatase inhibitor letrozole. Gynecol Oncol 95:796–771
Maluf FC et al (2001) Endometrial stromal sarcoma: objective response to letrozole. Gynecol Oncol 82:384–388
Smith IE, Dowsett M (2003) Aromatase inhibitors and breast cancer. New Engl J Med 348:2431–2442
Abdulhaq H, Geyer C (2008) Safety of adjuvant endocrine therapy in postmenopausal women with breast cancer. Amer J Clin Oncol 31(6):595–605
National Osteoporosis Foundation (2008) Clinician's guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation, Washington, DC
Osteoporosis (2004) ACOG practice bulletin no. 50. American College of Obstetricians and Gynecologists, Washington DC
Conflicts of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sylvestre, V.T., Dunton, C.J. Treatment of Recurrent Endometrial Stromal Sarcoma with Letrozole: A Case Report and Literature Review. HORM CANC 1, 112–115 (2010). https://doi.org/10.1007/s12672-010-0007-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-010-0007-9