Abstract
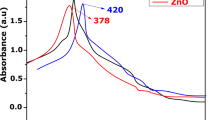
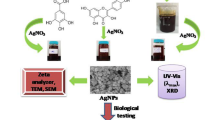
Up to date, fruit melanins have not been employed to synthesize nanoparticles (NPs). Crescentia alata, Randia echinocarpa, and Vitex mollis fruit are used in traditional medicine, and their soluble melanins (SMes) have high antioxidant activity. These SMes were used to prepare silver (green-AgNPs) and gold (green-AuNPs) NPs and evaluate their antioxidant capacity (DPPH and FRAP), activity against human pathogens (bacteria and the parasite Hymenolepis nana), and toxicity (Artemia salina assay). All SMes were useful to synthesize green-AgNPs but only V. mollis SMe for green-AuNPs. Infrared spectroscopy, dynamic light scattering, and transmission electron microscopy (TEM) showed that SMe is on the green-NPs surface. TEM showed 13–31-nm spherical green-AgNPs and 2–16-nm spherical and cylindrical green-AuNPs. Green-AgNPs showed higher antioxidant (FRAP = 3.4–725.4 µmol TE g−1; DPPH = 10.9–748.2 µmol TE g−1) and antibacterial (MIC = 1.85–15 µg mL−1; MBC = 3.7–30 µg mL−1) activities than the chemical-NPs. Moreover, the green-NPs (25 mg mL−1) were active against H. nana with the following times (min) (paralysis, death): green-AgNPs (10–40, 15–90) and V. mollis AuNPs (28, 40). The toxicity of the green-NPs (LC50 = 61.6 to > 1000 μg mL−1) depended on the SMe employed in the preparation. V. mollis NPs were the less toxic: minimal toxicity for the AgNPs (LC50 = 826.91 μg mL−1) and non-toxic for the AuNPs (LC50 > 1000 µg mL−1). Thus, the SMes are useful to obtain green-NPs of high stability that showed activities against human pathogens, suggesting their potential to be used as alternative health treatments.



Similar content being viewed by others
References
WHO (2014) Antimicrobial resistance. Global report on surveillance. https://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf;jsessionid=04D65BC1C5FC250876347EEEF578120F?sequence=1. Accessed 15 February 2019
El-Batal, A. I., El-Sayyad, G. S., El-Ghamry, A., Agaypi, K. M., Elsayed, M. A., & Gobara, M. (2017). Melanin-gamma rays assistants for bismuth oxide nanoparticles synthesis at room temperature for enhancing antimicrobial, and photocatalytic activity. Journal of Photochemistry and Photobiology B: Biology, 173, 120–139. https://doi.org/10.1016/j.jphotobiol.2017.05.030
Gurme, S. T., Aware, C. B., Surwase, S. N., Chavan, C. S., & Jadhav, J. P. (2019). Synthesis of melanin mediated silver nanoparticles from Aeromonas sp. SNS using response surface methodology: Characterization with the biomedical applications and photocatalytic degradation of brilliant green. Journal of Polymers and the Environment, 27(11), 2428–2438. https://doi.org/10.1007/s10924-019-01529-5
Patil, S., Sistla, S., Bapat, V., & Jadhav, J. (2018). Melanin-mediated synthesis of silver nanoparticles and their affinity towards tyrosinase. Applied Biochemistry and Microbiology, 54(2), 163–172. https://doi.org/10.1134/s0003683818020096
Roy S., & Rhim J. W. (2019). Melanin-Mediated synthesis of copper oxide nanoparticles and preparation of functional Agar/CuO NP nanocomposite films. Journal of Nanomaterials 2019, 1–10. https://doi.org/10.1155/2019/2840517
Wang, L. F., & Rhim, J. W. (2019). Isolation and characterization of melanin from black garlic and sepia ink. LWT - Food Science Technology, 99, 17–23. https://doi.org/10.1016/j.lwt.2018.09.033
Phongtongpasuk, S., Poadang, S., & Yongvanich, N. (2016). Environmental-friendly method for synthesis of silver nanoparticles from dragon fruit peel extract and their antibacterial activities. Energy Procedia, 89, 239–247. https://doi.org/10.1016/j.egypro.2016.05.031
Ortan, A., Fierascu, I., Ungureanu, C., Fierascu, R. C., et al. (2015). Innovative phytosynthesized silver nanoarchitectures with enhanced antifungal and antioxidant properties. Applied Surface Science, 358, 540–548. https://doi.org/10.1016/j.apsusc.2015.07.160
Markus, J., Wang, D., Kim, Y. J., Ahn, S., et al. (2017). Biosynthesis, characterization, and bioactivities evaluation of silver and gold nanoparticles mediated by the roots of Chinese herbal Angelica pubescens Maxim. Nanoscale Research Letters, 12(1), 46. https://doi.org/10.1186/s11671-017-1833-2
Devi, G. K., Kumar, K. S., Parthiban, R., & Kalishwaralal, K. (2017). An insight study on HPTLC fingerprinting of Mukia maderaspatna: Mechanism of bioactive constituents in metal nanoparticle synthesis and its activity against human pathogens. Microbial Pathogenesis, 102, 120–132. https://doi.org/10.1016/j.micpath.2016.11.026
Vanaraj, S., Jabastin, J., Sathiskumar, S., & Preethi, K. (2017). Production and characterization of bio-AuNPs to induce synergistic effect against multidrug resistant bacterial biofilm. Journal of Cluster Science, 28(1), 227–244. https://doi.org/10.1007/s10876-016-1081-0
Saini, P., Saha, S. K., Roy, P., Chowdhury, P., & Sinha Babu, S. P. (2016). Evidence of reactive oxygen species (ROS) mediated apoptosis in Setaria cervi induced by green silver nanoparticles from Acacia auriculiformis at a very low dose. Experimental Parasitology, 160, 39–48. https://doi.org/10.1016/j.exppara.2015.11.004
Phull, A.-R., Abbas, Q., Ali, A., Raza, H., et al. (2016). Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Future Journal of Pharmaceutical Sciences, 2(1), 31–36. https://doi.org/10.1016/j.fjps.2016.03.001
Vijayan S. R., Santhiyagu P., Singamuthu M., Kumari Ahila N., Jayaraman R., & Ethiraj K. (2014). Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Science World Journal 2014, 1–10. https://doi.org/10.1155/2014/938272
Cavallini C., Vitiello G., Adinolfi B., Silvestri B., et al. (2020) Melanin and melanin-like hybrid materials in regenerative medicine. Nanomaterials 10(8), 1518. https://doi.org/10.3390/nano10081518
Cuevas-Juárez, E., Yuriar-Arredondo, K. Y., Pío-León, J. F., Montes-Avila, J., et al. (2014). Antioxidant and α-glucosidase inhibitory properties of soluble melanins from the fruits of Vitex mollis Kunth, Randia echinocarpa Sessé et Mociño and Crescentia alata Kunth. Journal of Functional Foods, 9, 78–88. https://doi.org/10.1016/j.jff.2014.04.016
Montes-Avila, J., Ojeda-Ayala, M., López-Angulo, G., Pío-León, J. F., et al. (2018). Physicochemical properties and biological activities of melanins from the black-edible fruits Vitex mollis and Randia echinocarpa. Journal of Food Measurement and Characterization, 12(3), 1972–1980. https://doi.org/10.1007/s11694-018-9812-6
Pío-León, J. F., Montes-Avila, J., López-Angulo, G., Díaz-Camacho, S. P., et al. (2018). Melanins of Vitex mollis fruit with differences in water-solubility show high inhibition of carbohydrate digestive enzymes and antioxidant activity. Journal of Food Biochemistry, 42(3), e12509. https://doi.org/10.1111/jfbc.12509
Qi, C., Fu, L. H., Xu, H., Wang, T. F., Lin, J., & Huang, P. (2019). Melanin/polydopamine-based nanomaterials for biomedical applications. Science China-Chemistry, 62(2), 162–188. https://doi.org/10.1007/s11426-018-9392-6
Caldas M., Santos A. C., Rebelo R., Pereira I., et al. (2020) Electro-responsive controlled drug delivery from melanin nanoparticles. International Journal of Pharmaceutics 588, 119773. https://doi.org/10.1016/j.ijpharm.2020.119773
Montes-Avila, J., Díaz-Camacho, S. P., Willms, K., De-la-Cruz-Otero, M., d. C., et al. (2017). Bioguided study of the in vitro parasitocidal effect on adult Hymenolepis nana of the Psidium sartorianum (O. Berg Nied.) fruit methanol extract. Medicinal Chemistry Research, 26(11), 2845–2852. https://doi.org/10.1007/s00044-017-1983-x
Ibrahim, H. M. M. (2015). Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. Journal of Radiation Research and Applied Science, 8(3), 265–275. https://doi.org/10.1016/j.jrras.2015.01.007
Kuppusamy, P., Ichwan, S. J. A., Parine, N. R., Yusoff, M. M., Maniam, G. P., & Govindan, N. (2015). Intracellular biosynthesis of Au and Ag nanoparticles using ethanolic extract of Brassica oleracea L. and studies on their physicochemical and biological properties. Journal of Environmental Sciences, 29, 151–157. https://doi.org/10.1016/j.jes.2014.06.050
Qin, Y., Ji, X., Jing, J., Liu, H., Wu, H., & Yang, W. (2010). Size control over spherical silver nanoparticles by ascorbic acid reduction. Colloids and Surfaces A, 372(1–3), 172–176. https://doi.org/10.1016/j.colsurfa.2010.10.013
Dong, J., Carpinone, P. L., Pyrgiotakis, G., Demokritou, P., & Moudgil, B. M. (2020). Synthesis of precision gold nanoparticles using Turkevich method. Kona, 37, 224–232. https://doi.org/10.14356/kona.2020011
Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76. https://doi.org/10.1006/abio.1996.0292
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology, 28(1), 25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
CLSI. (2012). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard. Clinical and Laboratory Standards Institute.
Mangoni, M. L., Saugar, J. M., Dellisanti, M., Barra, D., Simmaco, M., & Rivas, L. (2005). Temporins, small antimicrobial peptides with leishmanicidal activity. Journal of Biological Chemistry, 280(2), 984–990. https://doi.org/10.1074/jbc.M410795200
Meyer, B., Ferrigni, N., Putnam, J., Jacobsen, L., Nichols, D., & McLaughlin, J. (1982). Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica, 45, 31–34. https://doi.org/10.1055/s-2007-971236
Abbott, W. S. (1925). (1987) A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 3(2), 302–303. https://doi.org/10.1093/jee/18.2.265a
Ben Tahar I., Fickers P., Dziedzic A., Ploch D., Skora B., & Kus-Liskiewicz M. (2019) Green pyomelanin-mediated synthesis of gold nanoparticles: Modelling and design, physico-chemical and biological characteristics. Microbial Cell Factories 18(1), 210. https://doi.org/10.1186/s12934-019-1254-2
Huang, X., & El-Sayed, M. A. (2010). Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. Journal of Advanced Research, 1(1), 13–28. https://doi.org/10.1016/j.jare.2010.02.002
Kumara, S. M., Sudipta, K. M., Jayanta, K., & Balasubramanya, S. (2015). The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Applied Nanoscience, 5(1), 73–81. https://doi.org/10.1007/s13204-014-0293-6
Wang, Z. J., Yu, N., Yu, W. J., Xu, H., et al. (2019). In situ growth of Au nanoparticles on natural melanin as biocompatible and multifunctional nanoagent for efficient tumor theranostics. Journal of Materials Chemistry B, 7(1), 133–142. https://doi.org/10.1039/c8tb02724b
Dhand, V., Soumya, L., Bharadwaj, S., Chakra, S., Bhatt, D., & Sreedhar, B. (2016). Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Materials Science and Engineering C, 58(1), 36–43. https://doi.org/10.1016/j.msec.2015.08.018
Islam, N. U., Jalil, K., Shahid, M., Rauf, A., et al. (2015). Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arabian Journal of Chemistry, 12(8), 2914–2925. https://doi.org/10.1016/j.arabjc.2015.06.025
de la Calle, I., Soto-Gomez, D., Perez-Rodriguez, P., & Lopez-Periago, J. E. (2019). Particle size characterization of sepia ink eumelanin biopolymers by SEM, DLS, and AF4-MALLS: A comparative study. Food Analytical Methods, 12(5), 1140–1151. https://doi.org/10.1007/s12161-019-01448-0
Sánchez, G. R., Castilla, C. L., Gómez, N. B., García, A., Marcos, R., & Carmona, E. R. (2016). Leaf extract from the endemic plant Peumus boldus as an effective bioproduct for the green synthesis of silver nanoparticles. Materials Letters, 185, 255–260. https://doi.org/10.1016/j.matlet.2016.07.115
Kumar, B., Smita, K., Debut, A., & Cumbal, L. (2016). Extracellular green synthesis of silver nanoparticles using Amazonian fruit araza (Eugenia stipitata McVaugh). Transactions of Nonferrous Metals Society of China, 26(9), 2363–2371. https://doi.org/10.1016/S1003-6326(16)64359-5
Muniyappan, N., & Nagarajan, N. S. (2014). Green synthesis of gold nanoparticles using Curcuma pseudomontana essential oil, its biological activity and cytotoxicity against human ductal breast carcinoma cells T47D. Journal of Environmental Chemical Engineering, 2(4), 2037–2044. https://doi.org/10.1016/j.jece.2014.03.004
Kelly, K. L., Coronado, E., Zhao, L. L., & Schatz, G. C. (2003). The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. The Journal of Physical Chemistry B, 107(3), 668–677. https://doi.org/10.1021/jp026731y
Noginov, M. A., Zhu, G., Bahoura, M., Adegoke, J., et al. (2007). The effect of gain and absorption on surface plasmons in metal nanoparticles. Appl Phys B-Lasers O, 86(3), 455–460. https://doi.org/10.1007/s00340-006-2401-0
Hulikere, M. M., Joshi, C. G., Danagoudar, A., Poyya, J., Kudva, A. K., & Dhananjaya, B. L. (2017). Biogenic synthesis of gold nanoparticles by marine endophytic fungus-Cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochemistry, 63, 137–144. https://doi.org/10.1016/j.procbio.2017.09.008
Dorosti, N., & Jamshidi, F. (2016). Plant-mediated gold nanoparticles by Dracocephalum kotschyi as anticholinesterase agent: Synthesis, characterization, and evaluation of anticancer and antibacterial activity. Journal of Applied Biomedicine, 14(3), 235–245. https://doi.org/10.1016/j.jab.2016.03.001
Mmola M., Roes-Hill M. L., Durrell K., Bolton J. J., et al. (2016) Enhanced antimicrobial and anticancer activity of silver and gold nanoparticles synthesised using Sargassum incisifolium aqueous extracts. Molecules 21(12), 1633. https://doi.org/10.3390/molecules21121633
Williams, D. N., Ehrman, S. H., Pulliam, T. R., & Holoman. (2006). Evaluation of the microbial growth response to inorganic nanoparticles. Journal of Nanbiotechnology, 4, 3. https://doi.org/10.1186/1477-3155-4-3
Burygin, G. L., Khlebtsov, B. N., Shantrokha, A. N., Dykman, L. A., Bogatyrev, V. A., & Khlebtsov, N. G. (2009). On the enhanced antibacterial activity of antibiotics mixed with gold nanoparticles. Nanoscale Research Letters, 4(8), 794–801. https://doi.org/10.1007/s11671-009-9316-8
Ramasamy, M., Lee, J. H., & Lee, J. (2017). Direct one-pot synthesis of cinnamaldehyde immobilized on gold nanoparticles and their antibiofilm properties. Colloids and Surfaces B: Biointerfaces, 160, 639–648. https://doi.org/10.1016/j.colsurfb.2017.10.018
Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., et al. (2005). The bactericidal effect of silver nanoparticles. Nanotechnology, 16(10), 2346–2353. https://doi.org/10.1088/0957-4484/16/10/059
Rai, M. K., Deshmukh, S. D., Ingle, A. P., & Gade, A. K. (2012). Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. Journal of Applied Microbiology, 112, 841–852. https://doi.org/10.1111/j.1365-2672.2012.05253.x
CLSI (2016) Performance standards for antimicrobial susceptibility testing. CLSI supplement M100S. Clinical and Laboratory Standards Institute
Chen, X., Yan, J.-K., & Wu, J.-Y. (2015). Characterization and antibacterial activity of silver nanoparticles prepared with a fungal exopolysaccharide in water. Food Hydrocolloid, 53, 69–74. https://doi.org/10.1016/j.foodhyd.2014.12.032
Kar P. K., Murmu S., Saha S., Tandon V., & Acharya K. (2014) Anthelmintic efficacy of gold nanoparticles derived from a phytopathogenic fungus, Nigrospora oryzae. PLoS One 9(1), e84693. https://doi.org/10.1371/journal.pone.0084693
Xie, S. Y., Pan, B. L., Shi, B. X., Zhang, Z. Z., et al. (2011). Solid lipid nanoparticle suspension enhanced the therapeutic efficacy of praziquantel against tapeworm. International Journal of Nanomedicine, 6, 2367–2374. https://doi.org/10.2147/ijn.s24919
Anderson, H. R., & Fairweather, I. (1995). Fasciola hepatica: Ultrastructural changes to the tegument of juvenile flukes following incubation in vitro with the deacetylated (amine) metabolite of diamphencthide. International Journal for Parasitology, 25(3), 319–333. https://doi.org/10.1016/0020-7519(94)00105-W
Tandon, V., Pal, P., Roy, B., Rao, H. S. P., & Reddy, K. S. (1997). In vitro anthelmintic activity of root-tuber extract of Flemingia vestita, an indigenous plant in Shillong, India. Parasitol Res, 83(5), 492–498. https://doi.org/10.1007/s004360050286
Arizmendi-Grijalva, A., Martinez-Higuera, A. A., Soto-Guzman, J. A., Martinez-Soto, J. M., et al. (2021). Effect on human vascular endothelial cells of Au nanoparticles synthesized from Vitex mollis. ACS Omega, 6(38), 24338–24350. https://doi.org/10.1021/acsomega.1c01506
Karthik, L., Kumar, G., Keswani, T., Bhattacharyya, A., Reddy, B. P., & Rao, K. V. B. (2013). Marine actinobacterial mediated gold nanoparticles synthesis and their antimalarial activity. Nanomedicine: Nanotechnology, Biology and Medicine, 9(7), 951–960. https://doi.org/10.1016/j.nano.2013.02.002
Acknowledgements
The authors acknowledge Dr. Rito Vega-Aviña for plant identification and Dr. Edgar Adán Valenzuela-García (Center of Studies of Foreign Languages of the Autonomous Occidental University) for the language assistance in the manuscript preparation.
Funding
This research was partially supported by the Autonomous University of Sinaloa (PROFAPI, “Programa de Fomento y Apoyo a Proyectos de Investigación,” PRO_A2_011) and the National Council for Science and Technology of Mexico (CONACyT, A1-S-32946).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Research Involving Humans and Animals Statement
None.
Informed Consent
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Montes-Avila, J., López-Angulo, G., Duarte-de-la-Peña, G. et al. Antioxidant, Antibacterial, and Antiparasitary Activities of Green Nanoparticles Synthesized Using Water-Soluble Melanins of Fruits. BioNanoSci. 12, 228–240 (2022). https://doi.org/10.1007/s12668-022-00940-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-022-00940-y