Abstract
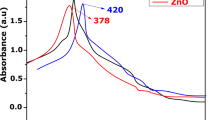
Functional metabolites are believed to possess different bioactivities; thus, three different stages of ripened fruit (green, yellow, red) of Capsicum annuum were collected from Chennai, Tamil Nadu, India, and were used in this study. The aqueous extract of Capsicum annuum was screened for various phytochemical compounds and subjected to TLC bioautography and antibacterial and antioxidant activities. The aqueous extract of yellow-colored Capsicum annuum showed the highest antibacterial activity against Pseudomonas aeruginosa and also showed the highest antioxidant activity. Furthermore, silver nanoparticles were synthesized using the aqueous extract and characterized by UV-Vis, FTIR, SEM, and AFM analysis. The silver nanoparticles were investigated for its bactericidal activity. The silver nanoparticles produced using the extract of green-colored Capsicum annuum showed the highest bactericidal activity which was evidenced by protein leakage assay.











Similar content being viewed by others
References
Samrot, A. V., Rohan, B., Kumar, D., Sahiti, K., Raji, P., & Samanvitha, S. K. (2016). Detection of antioxidant and antibacterial activity of Mangifera indica using TLC bio-autography. International Journal of Pharmaceutical Sciences and Research, 7(11), 4467–4472.
Sahiti, K., Raji, P., Rohan, B., Kumar, D., & Samrot, A. V. (2016). In vitro bioactivity screening of Desmostachya bipinnata. Research Journal of Pharmacy and Technology, 9(4), 361–364.
Amin, M. M., Sawhney, S. S., & Jassal, M. M. S. (2013). Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker Journal of Pharmacy and Pharmocology, 2(1), 001–005.
Ortega, M. H., Moreno, A. O., Navarro, M. D. H., Cevallos, G. C., Alvarez, L. D., & Mondragon, H. N. (2012). Antioxidant, antinociceptive, and anti-inflammatory effects of carotenoids extracted from dried pepper (Capsicum annuum L.). Journal of Biomedicine and Biotechnology, 524019, 1–10.
Bhutia, K. L., Meetei, N. G. T., & Khanna, V. K. (2016). In vitro regeneration of Dalle khursani, an important chilli cultivar of Sikkim, using various explants. Agrotechnology, 5(1), 142.
Wall, M. M., Waddell, C. A., & Bosland, P. W. (2001). Variation in β-carotene and total carotenoid content in fruits of Capsicum. Hortscience, 36, 746–749.
Breithaupt, D. E., & Schwack, W. (2000). Determination of free and bound carotenoids in paprika (Capsicum annuum L.) by LC/MS. European Food Research and Technology, 211(1), 52–55.
Cervantes-Paz, B., Yahia, E. M., Ornelas-Paz, J. J., Victoria-Campos, C. I., Junquera, I. V., Pérez-Martínez, J. D., & Escalante-Minakata, P. (2014). Antioxidant activity and content of chlorophylls and carotenoids in raw and heat-processed Jalapeño peppers at intermediate stages of ripening. Food Chemistry, 146, 188–196.
Giuffrida, D., Dugo, P., Torre, G., Bignardi, C., Cavazza, A., Corradini, C., & Dugo, G. (2013). Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chemistry, 140(4), 794–802.
Richins, R. D., Hernandez, L., Dungan, B., Hambly, S., Holguin, F. O., & O’Connell, M. A. (2010). A “green” extraction protocol to recover red pigments from hot Capsicum fruit. Hortscience, 45(7), 1084–1087.
Bielski, B. H., Richter, H. W., & Chan, P. C. (1975). Some properties of the ascorbate free radical. Annals of the New York Academy of Sciences, 258, 231–237.
Vanderslice, J. T., Higgs, D. J., Hayes, J. M., & Block, G. (1990). Ascorbic acid and dehydroascorbic acid content of foods-as-eaten. Journal of Food Composition and Analysis, 3, 105–118.
Hill, T. A., Ashrafi, H., Reyes-Chin-Wo, S., Yao, J., Stoffel, K., Maria-Jose, T., Kozik, A., Michelmore, R. W., & Deynze, A. V. (2013). Characterization of Capsicum annuum genetic diversity and population structure based on parallel polymorphism discovery with a 30K unigene Pepper GeneChip. PLoS One, 8(2), e56200. https://doi.org/10.1371/journal.pone.0056200.
Diaz-Perez, J. C. (2010). Bell pepper (Capsicum annum L.) grown on plastic film mulches: effects on crop microenvironment, physiological attributes, and fruit yield. Hortscience, 45(8), 1196–1204.
Group, T.S.C. (1991). Treatment of painful diabetic neuropathy with topical capsaicin. A multicenter, double-blind, vehicle-controlled study. Archives of Internal Medicine, 151, 2225–2229.
Spiller, F., Alves, M. K., Vieira, S. M., Carvalho, T. A., Leite, C. E., Lunardelli, A., Poloni, J. A., Cunha, F. Q., & de Oliveira, J. R. (2008). Anti-inflammatory effects of red pepper (Capsicum baccatum) on carrageenan- and antigen-induced inflammation. The Journal of Pharmacy and Pharmacology, 60, 473–478.
Arimboor, R., Natarajan, R. B., Menon, K. R., Chandrasekhar, L. P., & Moorkoth, V. (2015). Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: analysis and stability—a review. Journal of Food Science and Technology, 52(3), 1258–1271.
Govindarajan, V. S., & Sathyanarayana, M. N. (1991). Capsicum—production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Critical Reviews in Food Science and Nutrition, 29, 435–474.
Ramteke, C., Chakrabarti, T., Sarangi, B.K., Pandey, R.A. (2013). Synthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. Article ID 278925. https://doi.org/10.1155/2013/278925.
Azwanida, N. N. (2015). A review on the extraction methods use in medicinal plants, principle, strength and limitation. Medicinal and Aromatic Plants, 4, 196. https://doi.org/10.4172/2167-0412.1000196.
Jamal, A. K., Yaacob, W. A., & Laily, B. D. (2008). A chemical study on Phyllanthus reticulatus. Journal of Physical Science, 19(2), 45–50.
Haque, M. A., Hassan, M. M., Das, A., Begum, B., Ali, M. Y., & Morshed, H. (2012). Phytochemical investigation of Vernonia cinerea (Family: Asteraceae). Journal of Applied Pharmaceutical Science, 02(06), 79–83.
Ezhilan, B. P., & Neelamegam, R. (2012). GC-MS analysis of phytocomponents in the ethanol extract of Polygonum chinense L. Pharmacognosy Research, 4(1), 11–14.
Samrot, A. V., Sahiti, K., Raji, P., Rohan, B., Kumar, D., & Sharma, K. (2016). TLC bio-autography guided identification of antioxidant and antibacterial activity of Acacia senegal. Der Pharmacia Lettre, 8(9), 41–47.
Cimpoiu, D. C. J. (2006). Analysis of some natural antioxidants by thin-layer chromatography and high performance thin layer chromatography. Journal of Liquid Chromatography & Related Technologies, 7–8, 1125–1142.
Takao, T., Kitatani, F., Watanabe, N., Yagi, A., & Sakata, K. (1994). A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Bioscience, Biotechnology, and Biochemistry, 58, 1780–1783.
Mehra, S., Dubey, A., Mathew, J., & Mehra, M. (2015). Comparative assessment of antimicrobial activity of five extract of P. longum and P. nigrum against B. brevis, P. thailandensis, E. aerogenes and B. anthracis. JAAS Journal, 3(1), 14–21.
Kouassi, K. C., Koffi-Nevry, R., Nanga, Z. Y., da Silva, T. J. A., Yao, K., Lathro, J. S., Tano, K., & Loukou, G. Y. (2010). Assessing the antibacterial activity and phytochemical screening of Capsicum varieties from Côte d’Ivoire. Food, 4(1), 27–32.
Balashanmugam, P., & Kalaichelvan, T. P. (2015). Biosynthesis characterization of silver nanoparticles using Cassia roxburghii DC. aqueous extract, and coated on cotton cloth for effective antibacterial activity. International Journal of Nanomedicine, 10(Suppl 1: Challenges in biomaterials research), 87–97.
Benzie, F. F., & Strain, J. J. (1999). Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology, 299, 15–27.
Kharat, S. N., & Mendhulkar, V. D. (2016). Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Materials Science and Engineering C, 62, 719–724.
Rajathi, K., & Sridhar, S. (2013). Green synthesized silver nanoparticles from the medicinal plant Wrightia tinctoria and its antimicrobial potential. International Journal of ChemTech Research, 5(4), 1707–1713.
Sriranjani, R., Srinithya, B., Vellingiri, V., Brindha, P., Anthony, S. P., Sivasubramanian, A., & Muthuraman, M. S. (2016). Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. Journal of Molecular Liquids, 220, 926–930.
Logeswari, P., Silambarasan, S., & Abraham, J. (2015). Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. Journal of Saudi Chemical Society, 19(3), 311–317.
Ali, Z.A., Yahya, R., Sekaran, S.D., Puteh, R. (2016). Green synthesis of silver nanoparticles using apple extract and its antibacterial properties. Advances in Materials Science and Engineering. Article ID 4102196. https://doi.org/10.1155/2016/4102196.
Krishnan, R., Arumugam, V., & Vasaviah, S. K. (2015). The MIC and MBC of silver nanoparticles against Enterococcus faecalis—a facultative anaerobe. Journal of Nanomedicine & Nanotechnology, 6, 3.
Paredes, D., Ortiz, C., & Torres, R. (2014). Synthesis, characterization, and evaluation of antibacterial effect of Ag nanoparticles against Escherichia coli O157:H7 and methicillin resistant Staphylococcus aureus (MRSA). International Journal of Nanomedicine, 9, 1717–1729.
Koyyati, R., Nagati, V. B., Nalvothula, R., Merugu, R., Kudle, K. R., Marx, P., & Padigya, P. R. M. (2014). Antibacterial activity of silver nanoparticles synthesized using Amaranthus viridis twig extract. International Journal of Research in Pharmaceutical Sciences, 5(1), 32–39.
Maruthai, K., Vallayyachari, K., Ravibalan, T., Philip, S. A., Samrot, A. V., & Muthuraj, M. (2017). Antibacterial activity of the silver nanoparticles against Escherichia coli and Enterobacter sp. Progress in Bioscience and Bioengineering, 1(1), 29–35.
Gunalana, S., Sivaraja, R., & Rajendran, V. (2012). Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Progress in Natural Science: Materials International, 22(6), 693–700.
Gayathri, N., Gopalakrishnan, M., & Sekar, T. (2016). Phytochemical screening and antimicrobial activity of Capsicum chinense Jacq. International Journal of Advances in Pharmaceutics, 5(1), 12–20.
Abdelwahab, S. I., Abdul, A. B., Elhassan, M. M., Mohan, S., Ibrahim, M. Y., Mariod, A. A., AlHaj, N. A., & Abdullah, R. (2009). GC/MS determination of bioactive components and antibacterial properties of Goniothalamus umbrosus extracts. African Journal of Biotechnology, 8(14), 3336–3340.
Ibrahim, S. M., & Vaitheeswaran, M. (2016). GC-MS determination of bioactive compounds of Pongamia pinnata (L) Pierre (Fabaceae). World Journal of Pharmacy and Pharmaceutical Sciences, 5(5), 1046–1053.
Materska, M. (2015). Flavone C-glycosides from Capsicum annuum L.: relationships between antioxidant activity and lipophilicity. European Food Research and Technology, 240, 549–557.
Lee, Y., Howard, L. R., & Villalón, B. (1995). Flavonoids and antioxidant activity of fresh pepper (Capsicum annuum) cultivars. Journal of Food Science, 60, 473–476.
Benveniste, P. (2002). Sterol metabolism. In The Arabidopsis Book (pp. 1–31). American Society of Plant Biologists.
Ling, W. H., & Jones, P. J. (1995). Dietary phytosterols: a review of metabolism, benefits and side effects. Life Sciences, 57, 195–206.
Resendiz, S. H., Gombart, L., Cravatt, B. F., & Henriksen, S. J. (2001). Effect of oleamide on sleep and its relationship to blood pressure, body temperature and locomotor activity in rats. Experimental Neurology, 172(1), 235–243.
Igwe, K. K., Madubuike, A. J., Otuokere, I. E., Amaku, F. J., & Ikenga, C. (2016). GC-MS analysis for structural identification and bioactive compounds in methanolic leaf extract of Mallotus oppositifolius. International Journal of Scientific Research and Management, 4(5), 4123–4129.
Ogunlesi, M., Okiei, W., Ofor, E., & Osibole, A. E. (2009). Analysis of the essential oil from the dried leaves of Euphorbia hirta Linn (Euphorbiaceae), a potential medication for asthma. African Journal of Biotechnology, 8, 7042–7050.
Sutha, S., Devi, K. V., & Mohan, V. R. (2011). GC-MS determination of bioactive components of Erythropalum scandens Bl., Bijdr. Journal of Applied Pharmaceutical Science, 01(09), 170–173.
Casuga, F. P., Castillo, A. L., & Corpuz, M. J. A. T. (2016). GC–MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica (Blanco) (Moraceae) leaves. Asian Pacific Journal of Tropical Biomedicine, 6(11), 957–961.
Asghar, S. F., Rehman, H. U., Choudahry, M. I., & Rahman, A. U. (2011). Gas chromatography-mass spectrometry (GC-MS) analysis of petroleum ether extract (oil) and bio-assays of crude extract of Iris germanica. International Journal of Genetics and Molecular Biology, 3(7), 95–100.
Yoshiyuki, M., Nobukazu, T., Hisaaki, Y., Takayoshi, K., Megumi, O., Hirokazu, S., Horie, T., Norikazu, A., Masakazu, Y., Akio, M., Shonen, Y., & Kengo, S. (1996). Fatty acids selectively inhibit eukaryotic DNA polymerase activities in vitro. Biochimica et Biophysica Acta (BBA)-Gene Structure and expression, 1308(3), 256–262.
Jenecius, A. A., Uthayakumari, F., & Mohan, V. R. (2012). GC-MS determination of bioactive components of Sauropus bacciformis Blume (Euphorbiaceae). Journal of Current Chemical and Pharmaceutical Sciences, 2(4), 347–358.
Karim, A. M. P., Weam, A., Yousif, M., & Inas, O. (2017). GC-MS analysis and antimicrobial activity of Sudanese Brassica nigra L. (Brassicaceae) fixed oil. International Journal of Scientific Engineering and Applied Science, 3(1), 73–81.
Careaga, M., Fernández, E., Dorantes, L., Mota, L., Jaramillo, M. E., & Sanchez, H. H. (2003). Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. International Journal of Food Microbiology, 83, 331–335.
Torres, M. J., Chávez, G. A., & Chávez, R. E. (1999). Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: affinin and capsaicin. Journal of Ethnopharmacology, 64, 241–248.
Shotorbani, N. Y., Jamei, R., & Heidari, R. (2013). Antioxidant activities of two sweet pepper Capsicum annuum L. varieties phenolic extracts and the effects of thermal treatment. Avicenna Journal of Phytomedicine, 3(1), 25–34.
Mendoza-Reséndez, R., Núñez, N. O., Barriga-Castro, E. D., & Luna, C. (2013). Synthesis of metallic silver nanoparticles and silver organometallic nanodisks mediated by extracts of Capsicum annuum var. aviculare (Piquin) fruits. RSC Advances, 3(43), 20765–20771.
Singh, M., Mallick, A. K., Banerjee, M., & Kuma, R. (2016). Loss of outer membrane integrity in Gram-negative bacteria by silver nanoparticles loaded with Camellia sinensis leaf phytochemicals: plausible mechanism of bacterial cell disintegration. Bulletin of Materials Science, 39(37), 1871–1878.
Liu, Y. S., Chang, Y. C., & Chen, H. H. (2018). Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. Journal of Food and Drug Analysis, 26(2), 649–656.
Vidhu, V. K., Aromal, S. A., & Philip, D. (2011). Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 83, 392–397.
Agarwal, P., Bairwa, V. K., Kachhwaha, S., & Kothari, S. L. (2014). Green synthesis of silver nanoparticles using callus extract of Capsicum annuum L. and their activity against microorganisms. International Journal of Nanotechnology and Application, 4(5), 1–8.
Dakal, T. C., Kumar, A., Majumdar, R. S., & Yadav, V. (2016). Mechanistic basis of antimicrobial actions of silver nanoparticles. Frontiers in Microbiology, 7, 1831. https://doi.org/10.3389/fmicb.2016.01831.
Pal, S., Tak, Y. K., & Song, J. M. (2007). Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Applied and Environmental Microbiology, 27, 1712–1720.
Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., Kouri, J. B., Ram’ırez, J. T., & Yacaman, M. J. (2005). The bactericidal effect of silver nanoparticles. Nanotechnology, 16(10), 2346–2353.
Ghosh, B., & Ramamoorthy, D. (2010). Effects of silver nanoparticles on Escherichia coli and its implications. International Journal of Chemical Sciences, 8(5), S31–S40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 960 kb)
Rights and permissions
About this article
Cite this article
Samrot, A.V., Shobana, N. & Jenna, R. Antibacterial and Antioxidant Activity of Different Staged Ripened Fruit of Capsicum annuum and Its Green Synthesized Silver Nanoparticles. BioNanoSci. 8, 632–646 (2018). https://doi.org/10.1007/s12668-018-0521-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0521-8