Abstract
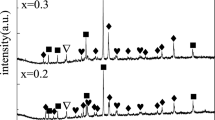
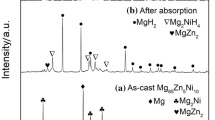
The filings of magnesium-rich Mg98.5Y1Zn0.5 alloy with different morphologies were prepared by using three kinds of files with arc (A), triangle (T), and flat (F) cross sections, respectively. Their microstructures, hydrogen storage properties, and mechanisms were investigated. The results reveal that these filings all present high storage capacity of about 7.0 wt% and excellent hydrogen sorption kinetics. However, the hydrogen ab/desorption properties of these filings exhibit obvious differences, which is closely related with their microscopic morphologies. Among them, the filings prepared by F file possess the best hydrogen sorption properties due to their higher saw teeth density and more dislocations induced through F file relative to those by A and T files. First-principles calculations reveal that the YH2 not only weakens the H–H and Mg–H bond strength, but also reduces the recombination energy of H atoms from MgH2 surface and the dissociation energy of H2 molecule on Mg surface during the hydrogenation/dehydrogenation of Mg–Y–Zn alloy.













Similar content being viewed by others
References
Sun Y H, Shen C Q, Lai Q W, Liu W, Wang D W, and Aguey Zinsou K F, Energy Storage Mater 10 (2018) 168.
Mohtadi R, and Orimo S I, Nat Rev Mater 2 (2017) 1.
Aschlapbach L, and Zuttel A, Nature 414 (2001) 353.
Yu X B, Tang Z W, Sun D L, Ouyang L Z, and Zhu M, Prog Mater Sci 88 (2017) 1.
Rusman N A A, and Dahari M, Int J Hydrogen Energy 41 (2016) 12108.
Prabhukhot P R, Wagh M M, and Gangal A C, Adv Energy Power 4 (2016) 11.
Xie X B, Chen M, Hu M M, Wang B L, Yu R H, and Liu T, Int J Hydrogen Energy 44 (2019) 10694.
Chen X Y, Chen R R, Ding X, Fang H Z, Li X Z, Ding H S, Su Y Q, Guo JJ, and Fu H Z, Energy 166 (2019) 587.
Hou X J, Wang Y, Yang Y L, Hu R, Yang G, Feng L, and Suo G Q, Energy 188 (2019) 116081.
Ouyang L Z, Cao Z J, Wang H, Hu R Z, and Zhu M, J Alloys Compd 691 (2017) 422.
Ren L, Zhou S, and Ou XM, Energy 209 (2020) 118482.
Xie X B, Hou C X, Chen C G, Sun X Q, Peng Y, Zhang Y P, Yu R H, Wang B, and Du W, Energy 211 (2020) 118959.
Zhang J, Yan S, and Qu H, Int J Hydrogen Energy 43 (2018) 1545.
Zhong H C, Huang Y S, Du Z Y, Lin H J, Lu X J, Cao C Y, Chen J H, and Dai L Y, Int J Hydrogen Energy 45 (2020) 27404.
Dou B L, Zhang H, Cui G M, He M X, Ruan C J, Wang Z L, Chen H S, Xu Y J, Jiang B, and Wu C F, Energy 167 (2019) 1097.
Yong H, Guo S H, Yuan Z M, Qi Y, Zhao D L, and Zhang Y H, J Mater Sci Technol 51 (2020) 84.
Zhang J, Huang Y N, Mao C, and Peng P, J Alloys Compd 538 (2012) 205.
Zhang J, Yan S, Xia G L, Zhou X J, Lu X Z, Yu L P, Yu X B, and Peng P, J Magnes Alloys 9 (2021) 647.
Ismail M, Energy 79 (2015) 177.
Chen L, Hu C Y, and Liu F, RSC Adv 9 (2019) 4445.
Luo F P, Wang H, Ouyang L Z, Zeng M Q, Liu J W, and Zhu M, Int J Hydrogen Energy 38 (2013) 10912.
Wu X, Zhang R, and Yang J, Phys Chem Chem Phys 18 (2016) 19412.
Shang C X, and Guo Z X, J Power Sources 129 (2004) 73.
Zhang W, Cheng Y, Han D, Han S M, Energy 93 (2015) 625.
Zhang C, Wang H, Ouyang L Z, Lin H J, and Zhu M, Prog Nat Sci Mater Int 27 (2017) 622.
Zou J X, Guo H, Zeng X Q, Zhou S, Chen X, and Ding W J, Int J Hydrogen Energy 38 (2013) 8852.
Luo Q, Li J D, Li B, Liu B, Shao H Y, and Li Q, J Magnes Alloys 7 (2019) 58.
Liu J W, Zou C C, Wang H, Ouyang L Z, and Zhu M, Int J Hydrogen Energy 38 (2013) 10438.
Zhang Y H, Wei X, Zhang W, Yuan Z M, Gao J L, Qi Y, and Ren H P, Int J Hydrogen Energy 45 (2020) 33832.
Zhou X J, Yao Y, Zhang J, Chen X M, Huang W Y, Pan J, Wang H P, and Weng MP, J Mater Sci Technol 70 (2021) 156.
Zhou X J, Xiong W Y, Zeng G, Xiao H C, Zhang J, Lu X Z, and Xiao X M, Mater Sci Eng A 805 (2021), 140596.
Liu L, Zhou X J, Yu S L, Zhang J, Shu X, and Su Z J, J Magnes Alloys (2021). Doi: https://doi.org/10.1016/j.jma.2020.09.023.
Huang S-J, Chiu C, Chou T-Y, and Rabkin E, Int J Hydrogen Energy 43 (2018) 4371.
Edalati K, Emami H, Staykov A, Smith D J, Akiba E, and Horita Z J, Acta Mater 99 (2015) 150.
Silva E P, Leiva D R, Pinto H C, Floriano R, Neves A M, and Botta W J, Int J Hydrogen Energy 43 (2018) 11085.
Wu Y, Solberg J K, and Yartys V A, J Alloys Compd 445 (2018) 178.
Song M Y, Kwon S, Bae J-S, and Hong S-H, Int J Hydrogen Energy 33 (2008) 1711.
Zhang Y H, Yuan Z M, Yang T, Qi Y, Guo S H, and Zhao D L, J Cent South Univ 23 (2016) 2754.
Zhang J, He L, Yao Y, Zhou X J, Yu L P, Lu X Z, and Zhou D W, Renew Energy 154 (2020) 1229.
Liu G, Wang Y, Jiao L, and Yuan H, Int J Hydrogen Energy 38 (2014) 3822.
Zhang L, Chen L, Fan X, Xiao X, Zheng J, and Huang X, J Mater Chem A 5 (2017) 6178.
Zhang M, Xiao X, Wang X, Chen M, Lu Y, and Chen L, Nanoscale 11 (2019) 7465.
Li Q, Li Y, Liu B, Lu X G, Zhang T F, and Gu Q F, J Mater Chem A 5 (2017) 17532.
Sun Y, Wang D B, Wang J M, Liu B Z, and Peng Q M, Int J Hydrogen Energy 44 (2019) 23179.
Li Q, Luo Q, and Gu Q F, J Mater Chem A 5 (2017) 3848.
Asselli A A C, Santos SF, and Huot J, J Alloys Compd 687 (2016) 586.
Silva E P, Leiva D R, Floriano R, Oliveira V B, Pinto H C, and Botta W J, Int J Hydrogen Energy 45 (2020) 5375.
Huang S-J, Rajagopal V, and Ali A N, Int J Hydrogen Energy 44 (2019) 1047-1058.
Zhang J, Yao Y, He L, Zhou X J, Yu L P, Lu X Z, and Peng P, Energy (2020) 119315.
Liu H, Ju J, Yang X W, Yan J L, Song D, Jiang J H, and Ma A B, J Alloys Compd 704 (2017) 509-517.
Zhou X J, Liu C M, Gao Y H, Jiang S N, and Chen Z Y, J Mater Eng Perform 27 (2018) 6237.
Zhu Y M, Morton A J, and Nie J E, Acta Mater 60 (2012) 6562.
Yong H, Wei X, Wang Y H, Guo S H, Yuan Z M, Qi Y, Zhao D L, and Zhang Y H, J Phys Chem Solids 144 (2020) 109516.
Han Z Y, Chen H P, and Zhou S X, Appl Surf Sci 394 (2017) 371.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51874049 and 51904036), the Science Research Project of Hunan Province Office of Education (No. 20A024), the Changsha Science and Technology Program Project (No. kq1907092), the Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation (No. 2019CL03), and the Research and Innovation Project of Graduate Students in Hunan Province (No. CX20200854).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yao, Y., Zhang, J., Zhou, X.J. et al. Filings Morphology-Dependent Hydrogen Storage Properties of Magnesium-Rich Mg–Y–Zn Alloy. Trans Indian Inst Met 74, 3171–3184 (2021). https://doi.org/10.1007/s12666-021-02379-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-021-02379-3