Abstract
The results of investigations (SEM/EDS and AAS) of a peat deposit, spanning 13,000 years of peat accumulation, are shown. The peat deposit is located in a region of shallow occurrence of Zn–Pb ores, near Tarnowskie Góry town, within the Cracow–Silesia district (southern Poland). Exploitation of lead, silver and iron during the medieval times (Twelfth and thirteenth century) was confirmed by historical documents whereas there are no unambiguous data showing that there was metal mining during the Romanian or earlier times in the region. The peat deposit is located within the influence of atmospheric Pb and Zn emission from a nearby Zn–Pb smelter. Two vertical peat profiles were investigated (120 and 140 cm depth of profile) showing variable concentrations of Zn up to 713 mg kg−1, Pb up to 317 mg kg−1, Cd up to 13 mg kg−1 and Tl up to 31 mg kg−1. The highest concentrations were recorded for the uppermost peat layers. SEM and EDS investigations revealed the occurrence of metalbearing, submicroscopic mineral components: Fe, Mn, Ti and Zn oxides and Zn and Pb carbonates. The top layer of the deposit contained Zn, Pb and Cd sulphides. The occurrence of aggregates of Au–Ag, Cu–Zn and Au–Ag–Cu alloys can be possibly related to pre-historical mining and smelting or be explained by geochemical transformations. The preservation of carbonates and oxides in the peat is discussed, indicating a generally neutral to alkaline peat water chemistry and maintenance of an oxidized environment in the fen.
Similar content being viewed by others
Introduction
Regions rich in precious metal deposits focused human attention from the dawn of history. In the vicinity of ores, located close to or exposed on the ground surface, human settlements were established, that main activity was ore mining and smelting. Starting from the early medieval period (eleventh and twelfth century), an intensive development in Cu, Sn, Ag, Pb, As and Fe ore mining took place in Europe, documented by historical data and confirmed using geochemical investigations (Merrington and Alloway 1994; Renberg et al. 2002; Monna et al. 2000).
One of the most important centre of early Pb–Ag and Fe mining in Europe was the Silesia–Cracow district (Southern Poland), where the mining activities were documented to occur since the late twelfth century (Molenda 1984). The limited amount of historical documents from that times cause a general lack of information about the exploitation of Pb, Ag and Fe before the twelfth century. A few reports from archaeological sites suggest that the metals mining in this region was probably much older, reaching the Hallstatt and roman culture times (Bindler et al. 2009). However, most of the proofs of early mining were lost during times of the later exploitation, especially intensive in the seventeenth century. This cause the need to look for alternative sources of evidences, including geochemical data. The identification of early anthropogenic sources of metal dispersion is difficult, because in such regions, natural geochemical aureoles of metals enrichment are formed, due to shallow occurrence of the ores. The most often investigated, metal enriched alluvium and soils, where the metal-bearing phases undergo long-term geochemical transformations, are difficult to interpret and to date (Maskall et al. 1996).
A promising source of information about the age and type of metal-bearing contaminants is provided by mires. Peat accumulating ecosystems are interesting objects for that type of investigations due to long-time period of peat accumulation, the possibility of precise dating and favorable conditions for metals preservation (Martínez-Cortizas et al. 2002; Forel et al. 2010).
For that reason vertical peat profiles are widely used to document changes in pollution fluxes in the past. Ombrotrophic, rain-fed peat bogs are regarded as most suitable (i.e., Bindler 2006; Coggins et al. 2006) but other mires, like minerotrophic fens and marshes were also suggested as a reliable material for retrospective investigations (Alfonso et al. 2001; Shotyk 2002; Monna et al. 2004).
Anthropogenically derived Pb, originated from ore processing in the Middle Bronze Age, was determined in peat deposits in France (Jouffroy-Bapicot et al. 2007). Metal exploitation during the Romanian times was recorded in peat bogs from France (Forel et al. 2010) and Spain (Monna et al. 2004). Fe ore exploitation in medieval period was recorded in peat profiles in Sweden (Bindler et al. 2011).
The importance of Pb emission from mining and reprocessing of Pb ores in South of Poland was highlighted by the investigations of peat bogs located in northern Poland (Vleeschouwer et al. 2009). In the vicinity of the shallow occurrences of Zn–Pb–Ag ores in Tarnowskie Góry region, small mires are located (Smieja-Król et al. 2010), significantly enriched in Pb and Zn, where the accumulated peat deposits constitute a potentially valuable archive of historical release of metal-bearing contaminants.
The aim of this paper was to use chemical and mineralogical analysis to depict processes responsible for Zn, Pb, Cd, Tl and Fe vertical distribution in a peat deposit located very close to the Tarnowskie Góry old mining region. The emphasis was put on the possible value of the peat to reconstruct the earliest signs of settlement and Pb–Ag mining. The impact of a nearby smelter, which is recently the most important heavy metal emitter in the region, on the peat geochemistry was also discussed.
Location and geological setting
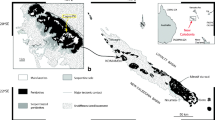
The peat deposit is located around 10 km north-east from Tarnowskie Góry and around 3 km east from a small village Żyglin, within the Silesia–Cracow district, Southern Poland (coordinates: N50°28′56.20″, E18°59′12.19″, 250 m from above mean sea level) (Fig. 1). Tarnowskie Góry and Żyglin are known from the medieval times for their importance as mining centres.
The peat deposit has a thickness between 30 and 120 cm and covers an area of around 2 ha. The peat deposit is a compact, undisturbed remnant of a minerotrophic fen underlined by sand sediments. The main feature of the peat is a presence of a continuous, horizontal dark layer, about 5–15 cm thick, rich in charcoal, located in the middle of the peat deposit. Above the layer, isolated fragments of charcoal were observed. The peat surface is now covered by 20 cm thick sand sediment. The morphology of the surrounding area indicates that the fen was probably supplied with waters originating from the Triassic carbonate rocks located north-west.
The investigated peat deposit is located on the border between catchments of Wisła and Odra rivers, in the drainage zone of Brynica River, close to drinking water intake “Bibiela”. In the 1980s, the fen was drained by a dense system of parallel ditches and forested. Nowadays the groundwater table level is below the peat layer for most of the year. The mean peat accumulation rate was established to be very low (0.01 mm/year) (Tudyka and Pazdur 2010) what is related with its high degree of humification; no plant remnants were possible to identify.
The peat deposit was dated by Tudyka and Pazdur (2010) in the purpose to reconstruct the time of emergence and development of early mining in the region. Their investigations showed that the peat comprise an extremely compact sediment spanning ca. 13,000 years, between ca. 12,000 bc and 700 ad.
The exploitation in the region ceased in the year 1917 after an abrupt flooding of iron mine “Bibiela”, located 3.7 km north of the peat deposit. Nowadays a main source of heavy metals pollution in the region is a zinc smelter “Miasteczko Śląskie” in operation since 1968, located 4.4 km upwind from the peat deposit.
The geological outline of the region is shown in Fig. 1. The Mesozoic sedimentary basement consists of limestone, dolomite and clay sediments of Middle Triassic age together with clay-silty sediments of Early Jurassic. The eroded carbonate Mesozoic rocks are locally covered by Pleistocene fluvioglacial sands and gravels, and Aeolian sands (Fig. 1).
Ore-bearing dolomite is the host rock for the Zn–Pb–Ag ore deposit. The ore is classified to belong to the Mississippi Valley Type (MVT), epigenetic, Early Cretaceous in age (Heijlen et al. 2003). The mineralogical assemblage includes mainly simple sulphides such as: sphalerite (ZnS), galena (PbS), pyrite and marcasite (FeS2) and grenockite (CdS), containing traces of Ag, As, Tl, Sb, Ba and Ge. During the Miocene and Pleistocene period, weathering processes lead to geochemical transformation of the primary ore and the formation of supergene, nonsulphide Zn–Pb–Fe deposits (Cabala 2001). Hydrated Fe sulphates formed first as a product of Fe sulphides oxidation. The unstable hydrated Fe sulphates transform further into hydrated Fe oxide-hydroxides forming limonites. Primary Zn sulphides were oxidized to unstable sulphates which in turn changed to stable Zn carbonates, mainly smithsonite (ZnCO3) and rarely monheimite (Zn,Fe)CO3. Advanced chemical weathering lead to the formation of Zn silicates, like hemimorphite Zn4Si2O7(OH)2·H2O. Galena is hardly soluble and its oxidation proceeds in a much slower rate in comparison to the other sulphides. The first product of galena oxidation was a Pb sulphate, anglesite, before it changed to the stable form, cerussite (PbCO3) (Cabala et al. 2008).
In the Silesia–Cracow district, in the vicinity of historical and present day mining and smelting sites, the environment and especially soils were heavily contaminated by metal-bearing phases hosting Zn, Pb, Fe, Mn, Cd, Tl, As and Sb (Bauerek et al. 2009; Cabala et al. 2008, 2011).
Materials and methods
Sampling
Two profiles (A and B) were sampled within a distance of about 0.5 m, at a site where the peat has the highest thickness. The profiles were sampled from the wall of a ditch after removal of over 20 cm portion of peat to get a fresh, undisturbed surface. Samples of a volume ~36 cm3 (3 × 3 × 6 cm) were taken every 10 cm, starting the sampling procedure from the bottom of the peat layer. The profiles were taken close (~1 m) to the profile dated by Tudyka and Pazdur (2010). Additionally a whole profile, around 10 cm in diameter, was sampled near the profiles A and B from the freshly uncovered surface, for mineral particles extraction by standard method (Wills 1985) and subsequent SEM investigation. The characteristic dark layer of a thickness of around 5 cm was present at 70 cm below ground surface at the sampling site.
Chemical analysis
Heavy metal contents (Zn, Pb, Fe, Mn, Cd and Tl) were analyzed in profile A and B by atomic absorption spectroscopy (AAS) using a SOLAAR M6 spectrometer. The samples were homogenized, dried and then 0.2 g of each sample was ground in an agate mortar. A mixture of pure acids was used for the mineralization of each sample: 40 % HF (2 ml), 65 % HNO3 (3 ml) and 35 % HCl (1 ml) and distilled water (2 ml). The mineralization was carried out at 110o C in a Milestone MLS 1200 microwave furnace according to Milestone recommendations (Milestone Microwave Digestion System 2012). To remove fluorosilicates, 50 ml of 4 % H3BO3 was added and each sample again mineralized. After around 20 min of the mineralization process, the resulting solution was transferred to a 100 ml flask and filtrated under pressure to plastic bottles using 0.45 μm filters.
Microscopic analysis
Mineralogical studies were carried out using an Environmental Scanning Electron Microscope (ESEM) Philips XL 30 with EDAX analyzer. Back Scattered Electron (BSE) images were obtained using a Centaurus attachment with a detector resolution of 0.3 Z. The accelerating voltage was 15 kV. EDS spectra analyses were processed using Phillips software. The investigated samples were fixed to carbon tapes (1 × 1.5 cm) placed on aluminium stubs. Specimens were cleaned in an air stream to remove loose mineral grains.
SEM investigations were carried out on raw samples of profile A, to determine the spatial relationship between organic matter and minerals, and on the separated mineral fraction of the additional profile. Small pieces of air-dried peat of profile A were directly placed on carbon tapes and carbon coated prior to analysis. The use of BSE imaging enabled easy detection of all inorganic particles as they appear lighter in the dark background composed of peat organic matter. The high contrast between the organic and inorganic constituents of the peat also enabled a semi-quantitative evaluation of the amount of mineral particles relative to organic matter. The separated mineral fraction from the additional profile was investigated using environmental mode with no carbon coating. Individual minerals and anthropogenic particles were identified based on their chemical composition, morphology and, in the case of profile A, spatial relations to organic matter.
All analyses were carried out in the laboratories of the Faculty of Earth Sciences, University of Silesia, Sosnowiec, Poland.
Results
Heavy metal concentration
The Zn concentration was comparable in the two analyzed profiles and varied between 10 and 713 mg kg−1 (Table 1). The highest values were recorded in the near-surface layers. In the deeper parts of the peat, Zn concentrations were relatively low, comparable to concentrations measured for uncontaminated soils. Second, lower maximum showed up in the bottom layers, where the Zn content was between 152–165 mg kg−1 at the depth of 130 cm.
Similar distribution was observed for Pb and Cd concentrations (Table 1). The highest values for Pb (195–317 mg kg−1) and Cd (7–13 mg kg−1) were recorded in the uppermost layers (profile A and B). Slightly enriched in Pb was also a sample in profile B at the depth of 130 cm (39 mg kg−1), corresponding with the increase in Zn content. Interesting are the relatively high Tl concentrations (from 5 to 20 mg kg−1) detected in the profiles at 50–60 and 110 and 130 cm. The highest Tl concentration of 31 mg kg−1 was recorded in the uppermost sample of profile A. The iron concentration was high, between 0.64 and 1.97 % but there was not observed any regularity in its distribution in the peat profile (Table 1). On the contrary, the Mn content was relatively low (50–153 mg kg−1).
Mineral composition by ESEM investigation
The minerals constituted a significant addition to the peat matter in most of the samples analyzed. The main feature of the peat mineralogy was the presence of Fe(hydro)oxides precipitates in the whole peat profile and its common occurrence in the uppermost (30–50 cm below ground surface) and, to lesser extent, in bottom samples (120–140 cm). Fe(hydro)oxides, found in the bottom peat layer, contained traces of vanadium, originating probably from the underlying fluvioglacial sands (Fig. 2a′ and 3a′). Fe (hydro)oxides were unevenly distributed among the peat organic matter; the morphology of the precipitates proves their in situ formation. They commonly formed infillings and incrustations of plant debris (Fig. 2d) and colomorphic coverings on organic tissues (Fig. 2c). There was observed an almost continuous change in Fe and Ca concentrations between organic tissues, only slightly enriched in Fe and Ca, organic matter rich in Fe with an addition of Ca and the appearance of Fe(hydro)oxides precipitates. In the middle of the profile, the EDS analyses showed a common enrichment of the organic tissues in Al and Ca and traces of iron.
BSE images of mineral particles. a Spheroidal grain (depth 20 cm), a′ authigenic spheroidal aggregate containing vanadium (depth 140 cm), b crystalline aggregates of authigenic barite in organic matter at depth 140 cm, c colomorphic forms of Fe minerals, 60 cm depth, d fistulous forms of Fe minerals 50 cm depth
EDS spectra of mineral grains, location of investigated minerals marked in Fig. 2. a Fe oxide with Zn and aluminosilicates, a′ Fe(hydro)oxide with vanadium addition, b Ba sulphates (barite), c Fe (hydro)oxides and Fe carbonates, d Fe (hydro)oxides
Authigenic barite constituted an easily found addition in the bottom peat samples. The mineral was seen in the form of rosettes of orthorhombic plates (Fig. 2b and b′). Barite prevailing in the upper part of the peat (Table 2) was of a distinctly different morphology: irregular and weathered, indicating allogenic origin.
The detrital minerals: quartz, feldspars and clay minerals were the dominant inorganic fraction in profile A. Zircon (ZrSiO4), REE phosphates (monazite and xenotime), ilmenite (FeTiO3, Figs. 4b, 5b) and Ti oxides, originating from the heavy mineral fraction of fluvioglacial sands, were often identified using BSE detector both in profile A and in the profile where the mineral fraction was separated (Table 2). The individual samples differed in relative abundance of the minerals analyzed. Samples from the depth 100–120 and 60–70 cm of profile A contained lower amount of detrital minerals. Relatively high amounts of detrital minerals were found at depth 80–90 cm and in the bottom sample (140 cm) where the addition of rounded sandy grains was seen.
EDS spectra of mineral grains, the location of the investigated minerals marked in Fig. 4. a Zn carbonate; b Fe–Ti oxides; b′ Pb carbonate or Pb oxide and Fe (hydro)oxide; c Au–Ag–Cu alloy; d Zn, Cu-bearing phases
Metal-bearing minerals and anthropogenic particles were relatively rare in the samples. Several spheroidal aluminosilicates and Fe oxides enriched in Zn (Figs. 2a, 3a) were found in the upper peat layer, at the contact with sand sediment. Zn carbonates were identified at the depth of~40 cm (Figs. 4a, 5a). Small particles of Pb carbonate were found at a depth of around 60 cm (Fig. 4b and Table 2). Together with the Zn and Pb bearing minerals, larger grains of Fe carbonates and dolomite were present.
Small plates (2–12 µm) of an Ag–Au and Ag–Au–Cu alloy (Fig. 4c) were an interesting finding at the depth of 70 cm, within the dark layer. The EDS spectrum (Fig. 5c) indicated a high Au content in the alloys. The porous appearance of these particles favours their prolong existence in the environment. Other phases, reach in Cu and Zn (Figs. 4d, 5d) were identified at the depth of around 40 cm in association with the Zn carbonates.
Discussion
Within the period of 12,000 years of the mire accumulation (Tudyka and Pazdur 2010), the peat had preserved different types of natural and anthropogenic metal releases to the environment. The initial metal enrichment, indigenous to the mire growth, was related with the weathering processes of the nearby Zn–Pb–Fe–Ag ores.
Chemical transformation of Fe-minerals, Ca–Mg carbonates and mobilisation of Fe, Zn, Pb, Ca and Mg ions is the essential effect of weathering of carbonate rocks containing Zn–Pb–Fe minerals. While migrating in soil, iron combines with other dissolved substances to form carbonates, oxides and aluminosilicates. Oxidation of sulphides, controlled by composition and properties of the parent rock and promoted by suitable humidity and redox potential of soils, results in the formation of new unstable minerals, mostly Fe, Mg and Zn sulphates. The sulphates weather at a fast rate forming stable forms such as: Zn carbonates, Pb carbonates, Fe-oxides and uncommon Zn silicates (hemimorfite) (Cabala 2001). In number of cases, the aggregates assume spherical shapes (Fig. 2a) and are associated with aluminosilicate spherulites. The new-born minerals tend to concentrate in top soil layers.
Capacity for heavy metals absorption increases in clay and organic matter rich soil layer. Number of metals (Zn, Cd, Mn) can be absorbed by secondary iron-bearing minerals, especially Fe (hydro)oxides. Investigation done (5 km near Zyglin) by Van Roy et al. (2006) indicated that 60 % of Zn is accumulated in Fe–Mn oxides. Iron leached from parent sulphides combine with other dissolved ore components and oxygen to form unstable hydrated sulphates. Most frequently among them are hydrated Fe oxides, which can develop their colomorphic and authomorphic platy-shaped crystals that range 50–100 µm in diameter.
In peaty soils the mobility of heavy metals is mainly controlled by the peat high cation exchange capacity. Therefore the main process of heavy metals immobilization in peat is their binding to organic matter (Brown et al. 2000 and references therein). From the heavy metals analyzed, Pb has the highest affinity to organic matter and is regarded as generally immobile in soils where there is an abundance of organic matter (Vile et al. 1999). Zn and Cd are less effectively retained by peat and were documented to migrate down the profile (Monna et al. 2004; Rausch et al. 2005). As Zn is an essential nutrient for plant, bioaccumulation processes probably influence its distribution in peat. In minerotrophic fens, such processes as sorption by inorganic phases and precipitation (mainly as sulphides and oxyhydroxides) additionally influence the heavy metals behaviour (Bendell-Young 1999; Syrovetnik et al. 2007). Iron oxide was found to be the dominating binding agent for Zn and Pb in a peat bog exposed to metal-rich groundwater (Syrovetnik et al. 2007). However, in that case the Fe content was up to 40 wt %. In anaerobic conditions Zn may also precipitate in the form of Zn sulphides at relatively low Zn concentrations in peat (100–200 mg kg−1) (Smieja-Król et al. 2010).
Minerals that form or are able to persist in soils for a prolonged period of time are good indicators of pH and Eh conditions. The presence of Zn and Pb carbonates indicates neutral to alkaline conditions to prevail in the peat. The lack of sulphides together with a common occurrence of Fe(hydro)oxides suggest aerobic conditions to dominate. This is rather unusual for such kind of sediments, as in waterlogged mires anaerobic conditions and dissolved organic substances, which act as reactants or ligands, typically promote the decomposition of Fe(hydro)oxides (Shotyk 1992).
In the studied profiles, the Fe(hydro)oxides can be indigenous to the peat, forming in mildly oxidizing conditions during the peat accumulation. This would explain the severe humification of the peat and correlate with the finding of numerous charcoal fragments within the 20–60 cm layer below ground surface.
An additional phenomenon that can lead to the precipitation of Fe(hydro)oxides is oxidation of the peat after deposition, related most probably with the development of the recent draining system and lowering of the ground water level due to mining activities and drinking water intake. In the investigated peat deposit, the more abundant precipitation of Fe(hydro)oxides in the upper (30–50 cm) and bottom peat layer (120–140 cm) is not correlated with increase of Fe content (Table 1) what can suggest a breakdown of iron-organic complexes and subsequent precipitation of the Fe(hydro)oxides. The oxidation proceeded due to interaction of oxygenated meteoric waters with the upper peat layers and, to lesser extent, interaction of bottom layers with oxidizing ground waters. As a consequence, the redox buffering capacity of the organic matter was lost.
The extent of meteoric water penetration marked by the common precipitation of Fe(hydro)oxides correlates with increased concentrations of analyzed trace metals. Therefore, it is suggested that the higher concentration of Zn, Pb and Cd in the upper peat layer resulted most probably from recent industry pollution, infiltrating into the peat with meteoric waters and is not indigenous to the peat. To a lesser extent the same phenomenon is observed for the bottom layers where an increase in Zn concentration can also be seen. Zn is rather weakly bound to organic matter (Linton et al. 2007) thus its mobility in peat is expected to be higher than that of Pb. The visibly higher Zn content was observed down to 40–50 cm while higher Pb concentration reached the 30–40 cm layer. Cd is enriched in the 30 cm layer below ground surface.
The Tl concentrations reveal an irregular distribution in the analysed profiles and were higher than that recorded for Cd, what is not a typical phenomenon for soils contaminated by Pb–Zn mining and smelting activities. The high concentrations of Tl probably indicate that Tl was supplied to the peat by the stream water and selectively absorbed by the peat organic matter. Tl contents in soils contaminated by mining and smelting Zn–Pb ores are variable and can be as high as 27 mg kg−1 (Lis et al. 2003). In the nearest vicinity of point emission sources, the Tl concentrations can locally reach the value of 350 mg kg−1 (Cabala et al. 2008). Mn concentration was very low throughout the peat profiles (Table 1). Soils developed on carbonaceous Triassic rocks contain generally ten times more of this element (Cabala 2009) than the analyzed peat. The study shows that Mn is not bound by the in situ precipitated Fe(hydro)oxides and has not a tendency to accumulate in the peat organic matter.
Spheroidal aluminosilicates and Fe oxides containing Zn (Figs. 2a, 3a) originated from the nearby smelter or are related with other high-temperature burning processes. Similar spheroidal aggregates, originated from Zn smelting processes, were identified in soils within a distance of a few kilometres from the emission source (Kucha et al. 1996; Kelebek et al. 2004). Barite, in the form of large crystalline grains, was often found in soils in the regions where the ores occur. Slightly melted barite aggregates are emitted by Zn smelters (Cabala 2009). Barite nanocrystals are common and abundant in the troposphere over the Silesia–Cracow region (Jabłońska et al. 2001).
Ag minerals were not described in the Silesia–Cracow Zn–Pb ores. Ag is present in Pb and Fe sulphides in the amount up to 180 mg kg−1 (Cabala 2009). The occurrence of Ag-bearing phases in the peat is rather unexpected and an ancient anthropogenic origin cannot be excluded.
Conclusion
-
1.
The presence of secondary metal-bearing phases, represented by Zn and Pb carbonates and Fe(hydro)oxides, indicates an alkaline pathway of geochemical alternation in the investigated peat profiles.
-
2.
The mire received water from the Triassic carbonate rocks what lead to peat alkalinization and resulted in the absence of the primary Fe, Pb and Zn sulphide and sulphate.
-
3.
The presence of the Zn–Cu, Ag–Au alloys might be related with an early mining and smelting activities but an unambiguous identification of such activities need to be confirmed by further studies.
-
4.
Heavy metals (Fe, Mn, Tl, Zn, Pb and Cd) in the peat originated from weathering of the shallow Zn–Pb ores. The significantly higher concentrations of Pb, Zn and Cd in the subsurface peat layer resulted from infiltration of pollution emitted recently by a nearby Zn–Pb smelter.
-
5.
The mineralogy of the peat upper layer together with the presence of metal-bearing phases in the overlying sands confirms the peat pollution by recent industry.
-
6.
Geochemical and mineralogical studies of heavy metals distribution in peat profiles are valuable in view of evaluation of environment contamination in regions located close to mining and smelting centres.
References
Alfonso S, Grousset F, Masse L, Tastet JPA (2001) European lead isotope signal recorded from 6,000 to 3,00 years BP in coastal marshes (SW France). Atmos Environ 35:3595–3605
Bauerek A, Cabala J, Smieja-Król B (2009) Mineralogical alterations of Zn–Pb flotation wastes of the Mississippi Valley Type ores (Southern Poland) and their impact on contamination of rain water runoff. Pol J Enivron Stud 18(5):781–788
Bendell-Young L (1999) Contrasting the sorption of Zn by oxyhydroxides of Mn and Fe, and organic matter along a mineral-poor to mineral-rich fen gradient. Appl Geochem 14:719–734
Bindler R (2006) Mired in the past–looking to the future: geochemistry of peat and the analysis of past environmental changes. Global Planet Change 53:209–221
Bindler R, Renberg I, Rydberg J, Andren T (2009) Widespread waterborne pollution in central Swedish lakes and the Baltic Sea from pre-industrial mining and metallurgy. Environ Pollut 157:2132–2141
Bindler R, Segerström U, Pettersson-Jensen I-M, Berg A, Hansson S, Holmström H, Olsson K, Renberg I (2011) Early medieval origins of iron mining and settlement in central Sweden: multiproxy analysis of sediment and peat records from the Norberg mining district. J Archaeol Sci 38:291–300
Brown PA, Gill SA, Allen SJ (2000) Metal removal from wastewater using peat. Water Res 34:3907–3916
Cabala J (2001) Development of oxidation in Zn-Pb deposits in Olkusz area. In: Piestrzyński A (ed) Mineral deposits at the beginning of the 21st century. Balkema, Amsterdam, pp 121–124
Cabała J. 2009. Heavy metals in ground soil environment of the Olkusz area of Zn–Pb ore exploitation. University of Silesia Trans, vol 2729, p 130 (in Polish, English summary)
Cabala J, Zogala B, Dubiel R (2008) Geochemical and geophysical study of historical Zn–Pb ore processing waste dump areas (Southern Poland). Pol J Enivron Stud 17(5):693–700
Cabala J, Rahmonov O, Jablonska M, Teper E (2011) Soil algal colonization and its ecological role in an environment polluted by past Zn–Pb mining and smelting activity. Water Air Soil Pollut 215(1–4):339–348
Coggins AM, Jennings SG, Ebinghaus R (2006) Accumulation rates of the heavy metals lead, mercury and cadmium in ombrotrophic peatlands in the west of Ireland. Atmos Environ 40:260–278
Forel B, Monna F, Petit C, Bruguier O, Losno R, Fluck P, Begeot C, Richard H, Bichet V, Chateau C (2010) Historical mining and smelting in the Vosges Mountains (France) recorded in two ombrotrophic peat bogs. J Geochem Explor 107:9–20
Heijlen W, Muchez PH, Banks DA, Schneider J, Kucha H, Keppens E (2003) Carbonate-hosted Zn–Pb deposits in Upper Silesia, Poland: origin and evolution of mineralizing fluids and constraints on genetic models. Econ Geol 98:911–932
Jabłońska M, Rietmeijer FJ, Janeczek J (2001) Fine grained barite in coal fly ash from the Upper Silesian Industrial Region. Environ Geol 40:941–948
Jouffroy-Bapicot I, Pulido M, Galop D, Monna F, Ploquin A, Baron S, Petit C, Lavoie M, Beaulieu J-L, de Richard H (2007) Environmental impact of early palaeometallurgy: pollen and geochemical analysis. Veg Hist Archaeobot 16:251–258
Kelebek S, Yörük S, Davis B (2004) Characterization of basic oxygen furnace dust and zinc removal by acid leaching. Miner Eng 17:285–291
Kucha H, Martens A, Ottenburgs R, De Vos W, Viaene W (1996) Primary minerals of Zn–Pb mining and metallurgical dumps and their environmental behavior at Plombières, Belgium. Environ Geol 27:1–15
Linton PE, Shotbolt L, Thomas AD (2007) Microbial Communities in Long-Term Heavy Metal Contaminated Ombrotrophic Peats. Water Air Soil Pollut 186:97–113
Lis J, Pasieczna A, Karbowska B, Zembrzuski W, Lukaszewski Z (2003) Thallium in soil and stream sediments of a Zn–Pb mining and smelting area. Environ Sci Technol 37:4569–4572
Martínez-Cortizas A, Garcia-Rodeja E, Pontevedra Pombal X, Nóvoa-Muñoz JC, Weiss D, Cherbulin A (2002) Atmospheric Pb deposition in Spain during the last 4,600 years recorded by two ombrotrophic peat bogs and implications for the use of peat as archive. Sci Total Environ 292:33–44
Maskall J, Whitehead K, Gee C, Thornton I (1996) Long-term mgration of metals at historical smelting sites. Appl Geochem 11:43–51
Merrington G, Alloway BJ (1994) The transfer and fate of Cd, Cu and Zn from two historic metalliferous mine sites in the UK. Appl Geochem 9:677–687
Milestone Microwave Digestion System (2012) http://www.milestonesci.com/images/assests/ethos-ez.pdf. Accessed May 2012
Molenda D (1984) Der polnische Bleibergbau und seine Bedeutung fur den europaischen Bleimarkt vom 12. bis 17. Jahrhundert. Der Anschnitt Beih 2:187–198
Monna F, Hamer K, Leveque J, Sauer M (2000) Pb isotopes as a reliable marker of early mining and smelting in the Northern Harz province (Lower Saxony, Germany). J Geochem Explor 68:201–210
Monna F, Galop D, Carozza L, Tual M, Beyrie A, Marembert F, Chateau C, Dominik J, Grousset FE (2004) Environmental impact of early Basque mining and smelting recorded in a high ash minerogenic peat deposit. Sci Total Environ 327:197–214
Rausch N, Ukonmaanaho L, Nieminen TM, Krachler M, Shotyk W (2005) Porewater Evidence of Metal (Cu, Ni, Co., Zn, Cd) Mobilization in an Acidic, Ombrotrophic Bog Impacted by a Smelter, Harjavalta, Finland and Comparison with Reference Sites. Environ Sci Technol 3921:8207–8213
Renberg I, Brannvall ML, Bindler R, Emteryd O (2002) Stable lead isotopes and lake sediments-a useful combination for the study of atmospheric lead pollution history. Sci Total Environ 292:45–54
Shotyk W (1992) Organic soils. In I. P. Martin and W. Chesworth (eds.), Weathering, soils, and Paleosols. Elsevier, Amsterdam, p 610
Shotyk W (2002) The chronology of anthropogenic, atmospheric Pb deposition recorded by peat cores in three minerogenic peat deposits from Switzerland. Sci Total Environ 292:19–31
Smieja-Król B, Fiałkiewicz-Kozieł B, Sikorski J, Palowski B (2010) Heavy metal behavior in peat-a mineralogical perspective. Sci Total Environ 408(23):5924–5931
Syrovetnik K, Malmstrom ME, Neretnieks I (2007) Accumulation of heavy metals in the Oostriku peat bog, Estonia: determination of binding processes by means of sequential leaching. Environ Pollut 147:291–300
Tudyka K, Pazdur A (2010) Radiocarbon dating of peat profile with metallurgy industry evidence. Geochronometria 35:3–9
Van Roy S, Vanbroekhoven K, Dejonghe W, Diels L (2006) Immobilization of heavy metals in the saturated zone by sorption and in situ bioprecipitation processes. Hydrometallurgy 83:195–203
Vile MA, Wieder RK, Novak M (1999) Mobility of Pb in Sphagnum-derived peat. Biogeochemistry 45:35–52
Vleeschouwer F, Fagel N, Cheburkin A, Pazdur A, Sikorski J, Mattielli N, Renson V, Fialkiewicz B, Piotrowska N, Le Roux G (2009) Anthropogenic impacts in North Poland over the last 1,300 years—a record of Pb, Zn, Cu, Ni and S in an ombrotrophic peat bog. Sci Total Environ 407:5674–5684
Wills BA (1985) Mineral processing technology. Pergamon Press, Oxford p 160
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cabala, J., Smieja-Król, B., Jablonska, M. et al. Mineral components in a peat deposit: looking for signs of early mining and smelting activities in Silesia–Cracow region (Southern Poland). Environ Earth Sci 69, 2559–2568 (2013). https://doi.org/10.1007/s12665-012-2080-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-2080-6