Abstract
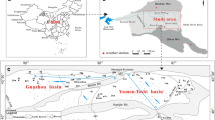
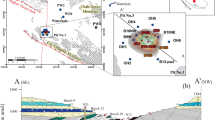
Extremely high concentrations of uranium (U) were discovered in shallow, groundwater-fed hyperalkaline soda lakes in Eastern Mongolia. A representative groundwater sample in this area is dilute and alkaline, pH = 7.9, with 10 mM TIC and 5 mM Cl−. In contrast, a representative lake water sample is pH ~ 10 with TIC and Cl− each more than 1,000 mM. Groundwater concentrations of U range from 0.03 to 0.43 μM L−1. Lake water U ranges from 0.24 to >62.5 μM, possibly the highest naturally occurring U concentrations ever reported in surface water. Strontium isotopes 87Sr/86Sr varied in groundwaters from 0.706192 to 0.709776 and in lakes 87Sr/86Sr varied from 0.708702 to 0.709432. High concentrations of U, Na, Cl−, and K correlate to radiogenic Sr in lake waters suggesting that U is sourced from local Cretaceous alkaline rhyolites. Uranium-rich groundwaters are concentrated by evaporation and U(VI) is chelated by CO −23 to form the highly soluble UO2(CO3) −43 . Modeled evaporation of lakes suggests that a U-mineral phase is likely to precipitate during evaporation.





Similar content being viewed by others
References
Agnerian J, Bocking K, Cox J, Heymann L (2007) Technical report on the Dornod Uranium Project, Mongolia. Prepared for Khan Resources Inc., Scott Wilson Roscoe Postle Associates Inc
Allison J, Brown D (1991) A geochemical assessment model for environmental systems: version 3.0 user’s manual. EPA/600/3-91/021. EPA, Athens
Appelo C, Postma D (1993) Geochemistry, Groundwater, and Pollution. A. A. Balkema, Rotterdam
Asikainen M, Kahlos H (1979) Anomalously high concentrations of uranium, radium and radon in water from drilled wells in the Helsinki region. Geochim Cosmochim Acta 43:1681–1686
Banner J, Kaufman J (1994) The isotopic record of ocean chemistry and diagenesis preserved in non-luminescent brachiopods from Mississippian carbonate rocks, Illinois and Missouri. Geol Soc Am Bull 106:1074–1082
Beadle L (1932) Scientific results of the Cambridge Expedition to the East African lakes 4. The waters of some East African lakes in relation to their fauna and flora. J Linn Soc Zool 38:128–138
Bethke C (1998) The geochemist’s workbench. University of Illinois
Bischoff J, Stine S, Rosenbauer R, Fitzpatrick J, Stafford T Jr (1993) Ikaite precipitation by mixing of shoreline springs and lake water, Mono Lake, California, USA. Geochim Cosmochim Acta 57:3855–3865
Brady P, Walther J (1989) Controls on silicate dissolution rates in neutral and basic pH solutions at 25°C. Geochim Cosmochim Acta 53:2823–2830
Clark D, Hobar D, Neu M (1995) Actinide carbonate complexes and their importance in actinide environmental chemistry. Chem Rev 95:25–48
Connell T, Dreiss S (1995) Chemical evolution of shallow groundwater along the northeast shore of Mono Lake, California. Water Resour Res 31:3171–3182
Council T, Bennett P (1993) Geochemistry of ikaite formation at Mono Lake, California: implications for the origin of tufa. Geology 21:971
Davis SN, Whittemore DO, Fabryka-Martin J (1998) Uses of chloride/bromide ratios in studies of potable water. Ground Water 36:338–350
Dodonov A (1979) Stratigraphy of the upper Pliocene-Quaternary deposits of Tadjikistan (Soviet Central Asia). Acta Geol Acad Sci Hung 22:63–73
Dodonov A (1991) Loess of Central Asia. GeoJournal 24:185–194
Dong H, Zhang G, Jiang H, Yu B, Chapman L, Lucas C, Fields M (2006) Microbial diversity in sediments of saline Qinghai lake, China: linking geochemical controls to microbial ecology. Microb Ecol 51:65–82
Drever J (1997) The geochemistry of natural waters: surface and groundwater environments. Simon and Schuster/A Viacom Company, Upper Saddle River
Eugster H (1980) Lake Magadi, Kenya, and its Pleistocene precursors. In: Nissenbaum A (ed) Hypersaline brines and evaporitic environments. Elsevier, Amsterdam, pp 195–232
Eugster H, Hardie L (1978) Saline lakes In: Lerman A (ed) Lakes: Chemistry, Geology, Physics. Springer-Verlag. New York, pp 237–293
Gosselin D, Sibray S, Ayers J (1994) Geochemistry of K-rich alkaline lakes, Western Sandhills, Nebraska, USA. Geochim Cosmochim Acta 58:1403–1418
Hardie L, Eugster H (1970) The evolution of closed-basin brines. Mineralogical Soc Am Spec Publ 3:273–290
Jones B, Van Denburgh A (1966) Geochemical influences on the chemical character of closed lakes. Symposium of Garda, Hydrology of Lakes and Reservoirs, Int Ass Sci Hydrol 70:435–446
Jones B, Eugster H, Rettig S (1977) Hydrochemistry of tthe Lake Magadi basin, Kenya. Geochim Cosmochim Acta 41:53–72
Katz J, Seaborg G, Morss L (1986) The Chemistry of the Actinide Elements. Chapman and Hall Ltd., Chicago
Ku T (1977) Uranium in open ocean: concentration and iotopic composition. Deep Sea Res 24:1005–1017
Langmuir D (1978) Uranium solution-mineral equilibria at low-temperatures with applications to sedimentary ore-deposits. Geochim Cosmochim Acta 42:547–569
Markwitz A, Barry B, Shagjjamba D (2008) PIXE analysis of sand and soil from Ulaanbaatar and Karakurum, Mongolia. Nucl Instrum Meth B 18:4010–4019
Mironov Y (2006) Uranium of Mongolia. Centre for Russian and Central EurAsian Mineral Studies, London
Nevin K, Lovley D (2000) Potential for nonenzymatic reduction of Fe(III) via electron shuttling in subsurface sediments. Appl Environ Microbiol 70:2472–2478
Parkhurst K, Appelo C (1999) Users’ guide to PHREEQC (Version 2)- A computer program for speciation, batch-reaction, one-dimenstional transport, and inverse geochemical calculations. USGS Water-Resources Investigations Report 99-4259: 310
Rogers D, Dreiss S (1995) Saline groundwater in Mono Basin, California.2. Long-term control of lake salinity by groundwater. Water Resour Res 31:3151–3169
Schmidt S, Moskal W, DM S, Howard-Williams C, Vincent W (1991) Limnological properties of Antarctic ponds during winter freezing. Antarct Sci 3:379–388
Simpson H, Trier R, Olsen C, Miller L, Melack J (1980) Fallout Plutonium in an alkaline, saline lake. Science 207:1071–1072
Stumm W, Morgan J (1996) Aquatic chemistry chemical, equilibria and rates in natural waters. John Wiley and Sons, Inc, New York
Tsurjimura M, Abe Y, Tanaka T, Shimada J, Higuchi S, Yamanaka T, Davaa G, Oyunbaatar D (2007) Stable isotopic and geochemical characteristics of groundwater in Kherlen River basin, a semi-arid region in eastern Mongolia. J Hydrol 333:47–57
Yamanaka T, Tsurjimura M, Oyunbaatar D, Davaa G (2005) Isotopic variation of precipitation over eastern Mongolia and its implication for the atmospheric water cycle. J Hydrol 333:21–34
Ying F, Duffy C, Oliver D (1997) Density-driven groundwater flow in closed desert basins: field investigations and numerical experiments. J Hydrol 196:139–184
Zhang Y, Tetsuo O, Kazuyoshi S (2005) Seasonal and inter-annual variation of snow cover surface sublimation in sourthern Siberia and north-eastern Mongolia. Nippon Kisho Gakkai Taikai Koen Yokoshu 87:350
Acknowledgments
We thank the US Student Fulbright Program and the Jackson School of Geosciences at The University of Texas at Austin for financial support. We also thank Todd Housh, Laura Heister and Larry Mack and the Central Geological Laboratory in Ulaanbaatar for sample analysis, and Jay Banner and John Sharp for insight into isotope geochemistry and hydrogeology. Finally, we would like to thank the anonymous reviewer who helped improve this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Linhoff, B.S., Bennett, P.C., Puntsag, T. et al. Geochemical evolution of uraniferous soda lakes in Eastern Mongolia. Environ Earth Sci 62, 171–183 (2011). https://doi.org/10.1007/s12665-010-0512-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-010-0512-8