Abstract
Introduction
Resistance to commonly used antibiotics against Helicobacter pylori (H. pylori) is increasing rapidly leading to lower success of traditional triple therapy to eradicate H. pylori infection. So, search for a new regimen as the first-line therapy of H. pylori infection is needed.
Aim
In this study, we compared the efficacy of 14-day concomitant therapy and 14-day triple therapy for the eradication of H. pylori infection.
Method
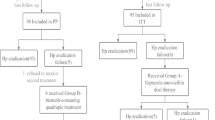
In this open-labeled prospective trial, patients with H. pylori infection were randomized to concomitant therapy (pantoprazole 80 mg, amoxicillin 2000 mg, clarithromycin 1000 mg, and metronidazole 1000 mg daily in divided doses) and triple therapy (pantoprazole 80 mg, amoxicillin 2000 mg, and clarithromycin 1000 mg daily in divided doses). Duration of treatment was 14 days. Gastric biopsy was done 10–12 weeks after completion of therapy to confirm H. pylori eradication.
Result
The eradication rate achieved with the concomitant therapy was significantly greater than that obtained with the triple therapy. Per-protocol eradication rates of concomitant and triple therapy were 77% and 58.3% (p = 0.028), respectively. Intention-to-treat eradication rates of concomitant and triple therapy were 70.1% and 49.3% (p = 0.013), respectively. Both the treatment regimens were well tolerated.
Conclusion
Although the rate of eradication of H. pylori infection with concomitant therapy was higher than that with triple therapy, the rate of concomitant therapy was still less than expected.


Similar content being viewed by others
References
Talebi A, Abadi B, Kusters JG. Management of Helicobacter pylori infections. BMC Gastroenterol. 2016;16:94.
Adlekha S, Chadha T, Krishnan P, Sumangala B. Prevalence of helicobacter pylori infection among patients undergoing upper gastrointestinal endoscopy in a medical college hospital in Kerala, India. Ann Med Health Sci Res. 2013;3:559–63.
Sodhi JS, Javid G, Zargar SA, et al. Prevalence of Helicobacter pylori infection and the effect of its eradication on symptoms of functional dyspepsia in Kashmir, India. J Gastroenterol Hepatol. 2013;28:808–13.
Thirumurthi S, Graham DY. Helicobacter pylori infection in India from a western perspective. Indian J Med Res. 2012;136:549–62.
Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5.
Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449–61.
Fock KM, Graham DY, Malfertheiner P. Helicobacter pylori research: historical insights and future directions. Nat Rev Gastroenterol Hepatol. 2013;10:495–500.
Malfertheiner P, Mégraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV Florence Consensus Report. Gut. 2012;61:646–64.
Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51–69.
Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587–600.
Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88:33–45.
Gehlot V, Mahant S, Mukhopadhyay AK, et al. Antimicrobial susceptibility profiles of Helicobacter pylori isolated from patients in North India. J Glob Antimicrob Resist. 2016;5:51–6.
Pandya HB, Agravat HH, Patel JS, Sodagar NR. Emerging antimicrobial resistance pattern of Helicobacter pylori in central Gujarat. Indian J Med Microbiol. 2014;32:408–13.
Thyagarajan SP, Ray P, Das BK, et al. Geographical difference in antimicrobial resistance pattern of Helicobacter pylori clinical isolates from Indian patients: Multicentric study. J Gastroenterol Hepatol. 2003;18:1373–8.
Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;12:CD008337.
Yeo YH, Shiu S-I, Ho HJ, et al. First-line Helicobacter pylori eradication therapies in countries with high and low clarithromycin resistance: a systematic review and network meta-analysis. Gut. 2017;67:20–7.
Roy AD, Deuri S, Dutta UC. The diagnostic accuracy of rapid urease biopsy test compared to histopathology in implementing “test and treat” policy for Helicobacter pylori. Int J Appl Basic Med Res. 2016;6:18–22.
Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med. 2015;3:9.
Sharma S, Mittal G, Agarwal RK, Ahuja V, Gupta R, Ahmad S. Comparison of different diagnostic methods of Helicobacter pylori in dyspeptic patients of a tertiary care hospital of Uttarakhand, India. Natl J Lab Med. 2016;5:MO06–10.
Choudhury G, Mohindra S. Epidemiology of Helicobacter pylori in India. Indian J Gastroenterol. 2000;19 Suppl 1:S3–6.
Lee HJ, Kim JI, Lee JS, et al. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol. 2015;21:351–9.
Lin L-C, Hsu T-H, Huang K-W, Tam K-W. No bismuth concomitant quadruple therapy for Helicobacter pylori eradication in Chinese regions: a meta-analysis of randomized controlled trials. World J Gastroenterol. 2016;22:5445–53.
Kumar D, Ahuja V, Dhar A, Sharma MP. Randomized trial of a quadruple-drug regimen and a triple-drug regimen for eradication of Helicobacter pylori: long-term follow-up study. Indian J Gastroenterol. 2001;20:191–4.
Pai CG, Thomas CP, Biswas A, Rao S, Ramnarayan K. Quadruple therapy for initial eradication of Helicobacter pylori in peptic ulcer: comparison with triple therapy. Indian J Gastroenterol. 2003;22:85–7.
Das R, Sureshkumar S, Sreenath GS, Kate V. Sequential versus concomitant therapy for eradication of helicobacter pylori in patients with perforated duodenal ulcer: a randomized trial. Saudi J Gastroenterol. 2016;22:309–15.
Ashokkumar S, Agrawal S, Mandal J, Sureshkumar S, Sreenath GS, Kate V. Hybrid therapy versus sequential therapy for eradication of Helicobacter pylori: a randomized controlled trial. J Pharmacol Pharmacother. 2017;8:62–7.
Gopal R, Elamurugan TP, Kate V, Jagdish S, Basu D. Standard triple versus levofloxacin based regimen for eradication of Helicobacter pylori. World J Gastrointest Pharmacol Ther. 2013;4:23–7.
Nasa M, Choksey A, Phadke A, Sawant P. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized study. Indian J Gastroenterol. 2013;32:392–6.
Javid G, Zargar SA, Bhat K, et al. Efficacy and safety of sequential therapy versus standard triple therapy in Helicobacter pylori eradication in Kashmir India: a randomized comparative trial. Indian J Gastroenterol. 2013;32:190–4.
Heo J, Jeon SW, Jung JT, et al. A randomised clinical trial of 10-day concomitant therapy and standard triple therapy for Helicobacter pylori eradication. Dig Liver Dis. 2014;46:980–4.
Georgopoulos S, Papastergiou V, Xirouchakis E, et al. Nonbismuth quadruple “concomitant” therapy versus standard triple therapy, both of the duration of 10 days, for first-line H. pylori eradication. A randomized trial. J Clin Gastroenterol. 2013;47:228–32.
Campillo A, Ostiz M, Amorena E, Kutz M, La Iglesia M. Quadruple concomitant non-bismuth therapy vs. classical triple therapy as first line therapy for Helicobacter pylori infection. Med Clin (Barc). 2016;147:199–201.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SKJ, MKM, KS, PJ, SP, and RR declare that they have no conflict of interest.
Ethics statement
The study was performed conforming to the Helsinki declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jha, S.K., Mishra, M.K., Saharawat, K. et al. Comparison of concomitant therapy versus standard triple-drug therapy for eradication of Helicobacter pylori infection: A prospective open-label randomized controlled trial. Indian J Gastroenterol 38, 325–331 (2019). https://doi.org/10.1007/s12664-019-00949-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-019-00949-4