Abstract
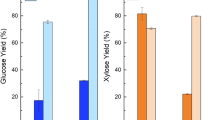
The efficient transformation of lignocellulosic biomass into fermentable sugars is essential for building bioeconomies. Sugarcane is an important agricultural crop in a number of Latin American countries, including Brazil and Argentina. Herein culms from two different sugarcane (SC384 and SC724) and two energy cane varieties (EC3116 and EC3118) bred in Argentina were evaluated for sustainable production of second-generation biofuels and green chemicals. Changes in the biomass crystallinity, structure, and morphology introduced by pretreatments were investigated using X-ray diffraction (DRX), confocal laser scanning microscopy (CLSM), and scanning electron microscopy (SEM) techniques. Enzymatic hydrolysis yields of untreated and pretreated sugarcane and energy cane culms were determined and correlated with physical analyses and chemical composition characterizations. Overall, after combined acid and alkali pretreatment, enzymatic convertibility was highly efficient for all studied sugarcane and energy cane varieties, reaching over 97% of theoretical conversion yields. High crystallinity indices and crystallite sizes of pretreated culms and SEM results and CLSM were consistent with the removal of lignin, solubilization of hemicellulose, and amorphous parts of lignocellulose imprinted by the pretreatments. High potential of culms from sugarcane and energy cane varieties cultivated in Argentina for sustainable production of renewable lignocellulosic sugars and their transformation into green chemicals and fuels was demonstrated.
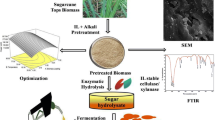
Graphic Abstract








Similar content being viewed by others
Data Availability
The data will be made available on request.
Abbreviations
- AFEX:
-
Ammonia fiber expansion
- ANOVA:
-
Analysis of variance
- CrI:
-
Crystallinity index
- DRX:
-
X-ray diffraction
- CLSM:
-
Confocal laser scanning microscopy
- EC:
-
Energy cane culm
- EC3116:
-
Energy cane variety INTA 05-3116
- EC3118:
-
Energy cane variety INTA 05-3118
- EHY:
-
Enzymatic hydrolysis yields
- GIMP:
-
GNU Image Manipulation Program
- HPLC:
-
High-performance liquid chromatography
- INTA:
-
National Institute of Agricultural Technology of Argentina
- LPMOs:
-
Lytic polysaccharide monooxygenases
- SC:
-
Sugarcane (Saccharum spp.) culm
- SC384:
-
Sugarcane variety LCP 85-384
- SC724:
-
Sugarcane variety NA 78-724
- SEM:
-
Scanning electron microscopy
References
Auxenfans, T., Terryn, C., Paës, G.: Seeing biomass recalcitrance through fluorescence. Sci. Rep. 7, 10 (2017). https://doi.org/10.1038/s41598-017-08740-1ï
Fatma, S., Hameed, A., Noman, M., Ahmed, T., Sohail, I., Shahid, M., Tariq, M., Tabassum, R.: Lignocellulosic biomass: a sustainable bioenergy source for future. Protein Pept. Lett. 25, 1–16 (2018). https://doi.org/10.2174/0929866525666180122144504
Kucharska, K., Rybarczyk, P., Hołowacz, I., Łukajtis, R., Glinka, M., Kamiński, M.: Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules. 23, 1–32 (2018). https://doi.org/10.3390/molecules23112937
Ranzi, E., Debiagi, P.E.A., Frassoldati, A.: Mathematical modeling of fast biomass pyrolysis and bio-oil formation. Note I: kinetic mechanism of biomass pyrolysis. ACS Sustain. Chem. Eng. 5, 2867–2881 (2017). https://doi.org/10.1021/acssuschemeng.6b03096
Schmatz, A.A., Salazar-Bryam, A.M., Contiero, J., Sant’Anna, C., Brienzo, M.: Pseudo-lignin content decreased with hemicellulose and lignin removal, improving cellulose accessibility, and enzymatic digestibility. Bioenergy Res. 14, 106–121 (2020). https://doi.org/10.1007/s12155-020-10187-8
Rinaldi, R., Woodward, R.T., Ferrini, P., Rivera, H.J.E.: Lignin-first biorefining of lignocellulose: the impact of process severity on the uniformity of lignin oil composition. J. Braz. Chem. Soc. 30, 479–491 (2019). https://doi.org/10.21577/0103-5053.20180231
Bordonal, R., de Carvalho, O., Lal, J.L.N., de Figueiredo, R., de Oliveira, E.B., la Scala, B.G.: Sustainability of sugarcane production in Brazil. A review. Agron. Sustain. Dev. 38, 1–23 (2018). https://doi.org/10.1007/s13593-018-0490-x
Bhalla, A., Cai, C.M., Xu, F., Singh, S.K., Bansal, N., Phongpreecha, T., Dutta, T., Foster, C.E., Kumar, R., Simmons, B.A., Singh, S., Wyman, C.E., Hegg, E.L., Hodge, D.B.: Performance of three delignifying pretreatments on hardwoods: hydrolysis yields, comprehensive mass balances, and lignin properties. Biotechnol. Biofuels 12, 213 (2019). https://doi.org/10.1186/s13068-019-1546-0
Matsuoka, S., Kennedy, A.J., dos Santos, E.G.D., Tomazela, A.L., Rubio, L.C.S.: Energy cane: its concept, development, characteristics, and prospects. Adv. Bot. 2014, 1–13 (2014). https://doi.org/10.1155/2014/597275
Borand, M.N., Karaosmanoğlu, F.: Biorefinery applications: a review effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: a review. Renew. Sustain. Energy 10, 1–23 (2018). https://doi.org/10.1063/1.5025876
Ogier, J.C., Ballerini, D., Leygue, J.P., Rigal, L., Pourquié, J.: Production d’éthanol à partir de biomasse lignocellulosique. Oil Gas Sci. Technol. 54, 67–94 (1999). https://doi.org/10.2516/ogst:1999004
Rani Singhania, R., Dixit, P., Kumar Patel, A., Shekher Giri, B., Kuo, C.-H., Chen, C.-W., di Dong, C.: Role and significance of lytic polysaccharide monooxygenases (LPMOs) in lignocellulose deconstruction. Bioresour. Technol. 335, 125261 (2021). https://doi.org/10.1016/j.biortech.2021.125261
Silveira, M.H.L., Morais, A.R.C., da Costa Lopes, A.M., Olekszyszen, D.N., Bogel-Łukasik, R., Andreaus, J., Ramos, P.: Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. ChemSusChem 8, 3366–3390 (2015). https://doi.org/10.1002/cssc.201500282
Li, X., Li, M., Pu, Y., Ragauskas, A.J., Klett, A.S., Thies, M., Zheng, Y.: Inhibitory effects of lignin on enzymatic hydrolysis: the role of lignin chemistry and molecular weight. Renew. Energy 123, 664–674 (2018). https://doi.org/10.1016/j.renene.2018.02.079
Das, P.: Novel pretreatment techniques for extraction of fermentable sugars from natural waste materials for bio ethanol production. Int. J. Environ. Sci. Nat. Resour. 7, 74–80 (2017). https://doi.org/10.19080/ijesnr.2017.07.555713
Chen, J., Adjallé, K., Barnabé, S., Perrier, M., Paris, J.: Mechanical and thermal pretreatment processes for increasing sugar production from woody biomass via enzymatic hydrolysis. Waste Biomass Valoriz. 10, 2057–2065 (2019). https://doi.org/10.1007/s12649-018-0217-x
Xu, L., Zhang, S.-J., Zhong, C., Li, B.-Z., Yuan, Y.-J.: Alkali-based pretreatment-facilitated lignin valorization: a review. Ind. Eng. Chem. Res. 59, 16923–16938 (2020). https://doi.org/10.1021/acs.iecr.0c01456
Sarkar, N., Ghosh, S.K., Bannerjee, S., Aikat, K.: Bioethanol production from agricultural wastes: an overview. Renew. Energy 37, 19–27 (2012). https://doi.org/10.1016/j.renene.2011.06.045
Li, J., Wei, X., Wang, Q., Chen, J., Chang, G., Kong, L., Su, J., Liu, Y.: Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 90, 1609–1613 (2012). https://doi.org/10.1016/j.carbpol.2012.07.038
Sindhu, R., Binod, P., Pandey, A.: Biological pretreatment of lignocellulosic biomass—an overview. Bioresour. Technol. 199, 76–82 (2016). https://doi.org/10.1016/j.biortech.2015.08.030
Hodgson-Kratky, K., Papa, G., Rodriguez, A., Stavila, V., Simmons, B., Botha, F., Furtado, A., Henry, R.: Relationship between sugarcane culm and leaf biomass composition and saccharification efficiency. Biotechnol. Biofuels 12, 247 (2019). https://doi.org/10.1186/s13068-019-1588-3
Rezende, C.A., de Lima, M., Maziero, P., Deazevedo, E., Garcia, W., Polikarpov, I.: Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 4, 54 (2011). https://doi.org/10.1186/1754-6834-4-54
Tsuchida, J.E., Rezende, C.A., de Oliveira-Silva, R., Lima, M.A., D’Eurydice, M.N., Polikarpov, I., Bonagamba, T.J.: Nuclear magnetic resonance investigation of water accessibility in cellulose of pretreated sugarcane bagasse. Biotechnol. Biofuels 7, 1–13 (2014). https://doi.org/10.1186/s13068-014-0127-5
Acevedo, A., Tejedor, M.T., Erazzú, L.E., Cabada, S., Sopena, R.: Pedigree comparison highlights genetic similarities and potential industrial values of sugarcane cultivars. Euphytica 213, 2–16 (2017). https://doi.org/10.1007/s10681-017-1908-2
Racedo, J., Gutiérrez, L., Perera, M.F., Ostengo, S., Pardo, E.M., Cuenya, M.I., Welin, B., Castagnaro, A.P.: Genome-wide association mapping of quantitative traits in a breeding population of sugarcane. BMC Plant Biol. 16, 142 (2016). https://doi.org/10.1186/s12870-016-0829-x
Lima, M.A., da Silva, H.K.P., Bragatto, J., Rezende, C.A., Bernardinelli, O.D., DeAzevedo, E.R., Gomez, L.D., McQueen-Mason, S.J., Labate, C.A., Polikarpov, I.: Effects of pretreatment on morphology, chemical composition and enzymatic digestibility of eucalyptus bark: a potentially valuable source of fermentable sugars for biofuel production—part 1. Biotechnol. Biofuels 6, 75 (2013). https://doi.org/10.1186/1754-6834-6-75
Rocha, G.J., de Martin, M., Soares, C., Souto Maior, I.B., Baudel, A.M., Moraes, H.M., de Abreu, C.A.: Dilute mixed-acid pretreatment of sugarcane bagasse for ethanol production. Biomass Bioenergy 35, 663–670 (2011). https://doi.org/10.1016/j.biombioe.2010.10.018
Segal, L., Creely, J.J., Martin, A.E., Conrad, C.M.: An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 29, 786–794 (1959). https://doi.org/10.1177/004051755902901003
Park, S., Baker, J.O., Himmel, M.E., Parilla, P.A., Johnson, D.K.: Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 3, 1–10 (2010). https://doi.org/10.1186/1754-6834-3-10
Langford, J.I., Wilson, A.J.C.: Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 11, 102–113 (1978). https://doi.org/10.1107/s0021889878012844
Santos, F.A., de Queiroz, J.H., Colodette, J.L., Manfredi, M., Queiroz, M.E.L.R., Caldas, C.S., Soares, F.E.F.: Otimização do pré-tratamento hidrotérmico da palha de cana-de-açúcar visando à produção de etanol celulósico. Quim. Nova 37, 56–62 (2014). https://doi.org/10.1590/S0100-40422014000100011
Cao, W., Sun, C., Liu, R., Yin, R., Wu, X.: Comparison of the effects of five pretreatment methods on enhancing the enzymatic digestibility and ethanol production from sweet sorghum bagasse. Bioresour. Technol. 111, 215–221 (2012). https://doi.org/10.1016/j.biortech.2012.02.034
Coletta, V.C., Rezende, C.A., da Conceição, F.R., Polikarpov, I., Guimarães, F.E.G.: Mapping the lignin distribution in pretreated sugarcane bagasse by confocal and fluorescence lifetime imaging microscopy. Biotechnol. Biofuels 6, 1–10 (2013). https://doi.org/10.1186/1754-6834-6-43
Chandel, A.K., Antunes, F.A.F., Anjos, V., Bell, M.J.V., Rodrigues, L.N., Polikarpov, I., de Azevedo, E.R., Bernardinelli, O.D., Rosa, C.A., Pagnocca, F.C., da Silva, S.S.: Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid-base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol. Biofuels 7, 63 (2014). https://doi.org/10.1186/1754-6834-7-63
Bensah, E.C., Mensah, M.: Chemical pretreatment methods for the production of cellulosic ethanol: technologies and innovations. Int. J. Chem. Eng. 1, 1–21 (2013). https://doi.org/10.1155/2013/719607
Zheng, Q., Zhou, T., Wang, Y., Cao, X., Wu, S., Zhao, M., Wang, H., Xu, M., Zheng, B., Zheng, J., Guan, X.: Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 8, 1–9 (2018). https://doi.org/10.1038/s41598-018-19517-5
Haque, M.A., Barman, D.N., Kang, T.H., Kim, M.K., Kim, J., Kim, H., Yun, H.D.: Effect of dilute alkali on structural features and enzymatic hydrolysis of barley straw (Hordeum vulgare) at boiling temperature with low residence time. J. Microbiol. Biotechnol. 22, 1681–1691 (2012). https://doi.org/10.4014/jmb.1206.06058
Brienzo, M., Ferreira, S., Vicentim, M.P., de Souza, W., Sant’Anna, C.: Comparison study on the biomass recalcitrance of different tissue fractions of sugarcane culm. Bioenergy Res. 7, 1454–1465 (2014). https://doi.org/10.1007/s12155-014-9487-8
Santos, M., Rezende, C.A., Bernardinelli, O.D., Pereira, N., Curvelo, A.A.S., Eduardo, R., Guimarães, F.E.G., Polikarpov, I.: Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind. Crops Prod. 113, 64–74 (2018). https://doi.org/10.1016/j.indcrop.2018.01.014
Bernardinelli, O.D., Lima, M.A., Rezende, C.A., Polikarpov, I., Ribeiro, E.: Quantitative 13 C MultiCP solid state NMR as a tool for evaluation of cellulose crystallinity index measured directly inside sugarcane biomass. Biotechnol. Biofuels 8, 1–11 (2015). https://doi.org/10.1186/s13068-015-0292-1
Santos, M., Brito, E., Eduardo, F., Guimaraes, G., Ribeiro, E., Paro, G., Henrique, E., de Oliveira, V., Pellegrini, A., Kumar, A., Henrique, M., Silveira, L., Polikarpov, I.: Multifaceted characterization of sugarcane bagasse under different steam explosion severity conditions leading to distinct enzymatic hydrolysis yields. Ind. Crops Prod. 139, 111542 (2019). https://doi.org/10.1016/j.indcrop.2019.111542
Simão, J.A., Carmona, V.B., Marconcini, J.M., Mattoso, L.H.C., Barsberg, S.T., Sanadi, A.R.: Effect of fiber treatment condition and coupling agent on the mechanical and thermal properties in highly filled composites of sugarcane bagasse fiber/PP. Mater. Res. 19, 746–751 (2016). https://doi.org/10.1590/1980-5373-MR-2015-0609
Donaldson, L., Williams, N.: Imaging and spectroscopy of natural fluorophores in pine needles. Plants 7, 1–16 (2018). https://doi.org/10.3390/plants7010010
Donaldson, L.: Softwood and hardwood lignin fluorescence spectra of wood cell walls in different mounting media. IAWA J. 34, 3–19 (2013). https://doi.org/10.1163/22941932-00000002
Thite, V.S., Nerurkar, A.S.: Valorization of sugarcane bagasse by chemical pretreatment and enzyme mediated deconstruction. Sci. Rep. 9, 1–14 (2019). https://doi.org/10.1038/s41598-019-52347-7
Chen, W.H., Tu, Y.J., Sheen, H.K.: Disruption of sugarcane bagasse lignocellulosic structure by means of dilute sulfuric acid pretreatment with microwave-assisted heating. Appl. Energy. 88, 2726–2734 (2011). https://doi.org/10.1016/j.apenergy.2011.02.027
Wilkinson, S., Smart, K.A., Cook, D.J.: A comparison of dilute acid- and alkali-catalyzed hydrothermal pretreatments for bioethanol production from brewers’ spent grains. J. Am. Soc. Brew. Chem. 72, 143–153 (2014). https://doi.org/10.1094/ASBCJ-2014-0327-02
Robak, K., Balcerek, M., Dziekońska-Kubczak, U., Dziugan, P.: Effect of dilute acid pretreatment on the saccharification and fermentation of rye straw. Biotechnol. Prog. 35, e2789 (2019). https://doi.org/10.1002/btpr.2789
Noparat, P., Prasertsan, P., O-Thong, S., Pan, X.: Dilute acid pretreatment of oil palm trunk biomass at high temperature for enzymatic hydrolysis. Energy Procedia. 79, 924–929 (2015). https://doi.org/10.1016/j.egypro.2015.11.588
Yang, J., Xu, H., Jiang, J., Zhang, N., Xie, J., Zhao, J., Wei, M.: Enhanced enzymatic hydrolysis and structure properties of bamboo by moderate two-step pretreatment. Appl. Biochem. Biotechnol. 193, 1011–1022 (2021). https://doi.org/10.1007/s12010-020-03472-x
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) via Grant 2015/13684-0, by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) via Grants 423693/2016-6 and 303988/2016-9, by Instituto Nacional de Tecnología Agropecuaria (INTA) via Grant PNAIyAV 2019-PE-E6-I114-001, and by Ministry of Science and Technology of Argentina via Grant PICT 2016-1670. This work was also supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) via a PhD fellowship to AOK. JMG has a fellowship from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). We thank the field team of INTA Sugarcane Breeding Program for its technical assistance.
Author information
Authors and Affiliations
Contributions
AOK: Formal analysis; Investigation; Methodology; Writing—original draft; VOAP: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing—original draft; MCES: Formal analysis; Investigation; Methodology; Writing—original draft; BDN: Supervision; Writing—original draft. JMG: Formal analysis; Investigation; Methodology; Writing—original draft; AA: Formal analysis; Investigation; Methodology; Writing—original draft. LEE: Formal analysis; Investigation; Methodology; Writing—original draft; IP: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Resources; Supervision; Validation; Writing—original draft; Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kane, A.O., Pellergini, V.O.A., Espirito Santo, M.C. et al. Evaluating the Potential of Culms from Sugarcane and Energy Cane Varieties Grown in Argentina for Second-Generation Ethanol Production. Waste Biomass Valor 13, 329–343 (2022). https://doi.org/10.1007/s12649-021-01528-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01528-5