Abstract
Purpose
An experiment was conducted to evaluate the potential use of a combination of food waste compost with sorbents as immobilizing agents to aid the phytostabilization of Ni-contaminated soil, using Lolium perenne L. (L. perenne).
Methods
The content of Ni in plants, i.e. total and extracted by 0.01 M CaCl2, was determined using the spectrophotometry method. Tests for the phytotoxicity of food waste compost were carried out using Phytotoxkit™ tests.
Results
The compost phytotoxicity tests showed that the growth of the roots of L. perenne was slightly stimulated for a compost concentration of 25%, showing the positive impact of this material on plant growth. The biomass of L. perenne in particular organs, Ni content and the properties of soil depended on the dose of a Ni contaminant and the type of mineral and organic mixture incorporated into the soil.
Conclusions
Ni accumulated predominantly in the roots of the L. perenne. The greatest increase pH was observed after compost with chalcedonite mixture was added to the soil. The application of mineral and organic mixture, containing compost from food wastes and chalcedonite as a soil amendment, tended to reduce the soil total and mobile fraction of Ni more in comparison to the un-amended soil.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of novelty
Problems concerning food waste management and disposal worldwide are currently among the most difficult and most topical issues in the organized waste management system. Apart from the least appropriate placing of food waste in landfills, the methods for disposal/recycling of food waste are the production of animal feed and composting. It is considered that the compost may be recycled for the removal of heavy metals from contaminated soils through the use of organic acids, microorganisms and nutrients found in composts. In this manuscript, we show that the mixture of compost from food waste and mineral sorbents (chalcedonite, halloysite), are a promising amendments for co-remediation methods of nickel contaminated soils. These novel soil amendments could be used for enhanced phytostabilization methods.
Introduction
Problems concerning food waste (FW) management and disposal worldwide are currently among the most difficult and most topical issues in the organized waste management system (WMS). The currently functioning food systems are very inefficient; for example, it is estimated that as much as half of the produced foods is wasted [1]. Another major problem is the removal of excess FW produced in urban areas [2]. In conclusion, over 40% of foods produced use a large amount of energy and valuable land and aquatic resources consequently become waste [3]. Significant and ever-increasing amounts of FW on a large scale are disposed of by placing this waste in landfills. Since a very important issue is the control of the excessive deposition of FW, increasing emphasis is being placed on improving recycling and disposal methods and on the searching for new ways of their efficient use.
FW are classified as organic wastes originating from various sources, e.g. from households, cafés or restaurants. It should be stressed that the incineration or deposition of FW are not economical methods, primarily due to the high water and organic substance content of these materials [4]. Apart from the least appropriate placing of FW in landfills, the methods for disposal/recycling of FW are the production of animal feed and composting [5].
Composting is an environmentally friendly, biochemical and natural method which requires a minimum external energy source [6,7,8,9]. There are several factors affecting the maturation of composting, including feed mixtures C:N ratio, aeration, moisture content, temperature, pH, land requirement, etc. Bioreactor composting systems have a big advantage over traditional/windrow composting systems: they require less space and ensure better control than windrow systems do. They are also are very efficient and ensure appropriate conditions for the complete decomposition of organic substances [10,11,12,13].
Composting in bioreactors converts the FW (organic waste) into a stable humus-like material that can be used as an organic fertilizer, soil stabilizer, soil amendment and promoter of crop growth [14,15,16]. Nevertheless, it is considered that the compost may be recycled for the removal of heavy metals from contaminated soils through the use of organic acids, microorganisms and nutrients found in composts.
Soil, as the top layer of the lithosphere, is the first recipient of all kinds of contaminants generated during anthropogenic activities [17,18,19]. The heavy metal content of the soil is stable and irreversible, which means that heavy metals are not decomposed (including biologically) [20], as is the case with most organic contaminants, which need to be either chemically/biologically immobilized or physically removed from the soil [21]. In order to eliminate heavy metals from contaminated soils, many remediation and ex-situ recovery methods are employed, including excavation, soil washing, and phytoremediation [22]. However, most conventional technologies used for remediation are expensive, greatly interfere with the other natural environment components and take a long time [23].
Environmentally friendly methods include phytostabilization, which is aimed at counteracting wind and water erosion as well as improving the physical properties of soils, and phytosanitary isolation of an area. Phytostabilization involves the use of plants to immobilize contaminants in the soil and to reduce their availability in the natural environment. The characteristic feature of xenobiotic immobilization is that it can take place as a result of metal absorption and accumulation in plant roots, adsorption on the surface of the roots or precipitation in the rhizosphere zone. Literature [24, 25] reports Lolium perenne L. to be a suitable species for the revegetation of metal-contaminated soils from metallurgical sites, mine tailings, and metalliferous wastes. Moreover, Smith and Bradshaw [26] defined this species as a facultative metallophyte, this plant produces high dry matter yields and well accumulates metals [27]. Additionally, Arienzo et al. [28] showed that a healthy vegetative cover using L. perenne could be installed on metal-polluted soils. Phytostabilization may be supported using a variety of additives, including composts and mineral sorbents [29]. Previous studies [30,31,32,33] have shown that individual materials used as additives aid phytostabilization. In this article, the authors recognized the impact of new amendments in the form of mineral and organic mixture on the process of aided phytostabilization in a soil artificially contaminated with Ni, enabling their use under in-situ conditions. The objectives of the present study were (i) to observe changes in the physico-chemical properties of the tested soil (ii) to compare the effectiveness of various soil mixtures of additives on various chemical properties of the plant–soil system.
Materials and Methods
Characterization of Soil and Composts
The soil used in the experiment was characterized by the following physico-chemical properties: grain-size distribution: fractions 2.0–0.05 mm 86.6 (%); fractions 0.05–0.002 mm 11.2 (%), fractions 0.002 mm 2.2 (%); pH 5.81; electrical conductivity (µS/cm) 87.21; hydrolytic acidity (mmol/kg) 31.21; sum of exchangeable bases, Ca2+, Mg2+, K+, Na+ (mmol/kg) 61.10; cation exchange capacity (mmol/kg) 94.20; base saturation (%) 65.20; total nitrogen (g/kg) 0.98; organic carbon (g.kg) 6.42; ammonia (mg/kg) 20.32; nitrate (V) (mg/kg) 2.01; extractable phosphorous (mg/kg) 43.20; extractable potassium (mg/kg) 8.72; extractable magnesium (mg/kg) 31.2; and Cu (mg/kg) 8.20.
According to our previous experiences the compost used in the experiment was prepared from 80% w/w of food waste and 20% w/w of wood pellets with a length of up to 40 mm and a diameter of 8 mm. Moreover, wood chips were used in order to raise the C:N ratio, because the compost emits an unpleasant odor if the C:N ratio is not in the appropriate range. Wood pellets were used as a bulking agent and carbon source in the production of the compost. The wood pellets were characterized by the content as specified by the supplier: C—50.1%; N—0.1%; with C:N ratio of—500:1; dry matter content—91.5%; bulk density—650 kg/m3; ash content < 0.7%. According to the scientific literature the C/N ratio is important for several aspects of composting but is particularly crucial for the development of microorganisms during composting because it provides the carbon and nitrogen source required for growth [34, 35]. The results of compost analysis are presented in Table 1.
Experimental Set-Up
The greenhouse experiment was carried out in 5.0 kg polyethylene (PE) pots containing soil contaminated with Ni, amended with the combination compost with mineral-based amendments (chalcedonite, halloysite) and vegetated with L. perenne plants. The pots were maintained under natural day/night conditions; during the day (14 h), the air temperature was 26 ± 3 °C and ∼ 10° lower (16 ± 2 °C) at night (10 h), with a relative humidity of 75 ± 5%. Simulated soil contamination with Ni (in the form of chemically pure aqueous solutions NiSO4·7H2O) was introduced in the following doses (mg/kg of soil): 0 (control), 150, 250, 350. The soil used in this study was sampled from a non-Ni-enriched site in an agricultural area from the sub-soil found in the layer between 10 and 30 cm below the surface. Stones, sticks and roots were manually removed. The soil texture was dominated by 2.0–0.05 mm (85%), 0.05–0.002 mm (15%), and 0.002 mm (1.8%) fractions. The soil was acidic (pH 5.6), contained 8.04 (g/kg) total organic carbon and was found to have a hydrolytic acidity of 34.21 (mmol/kg), sum of exchangeable bases (Ca2+, Mg2+, K+, Na+)—60.12 (mmol/kg), total nitrogen—1.02 (g/kg), organic carbon—7.32 (g/kg), extractable phosphorous (mg/kg)—30.12, extractable potassium (mg/kg)—7.32, extractable magnesium (mg/kg)—28.04, nickel—5.11 (mg/kg). Mineral-based amendments, i.e. chalcedonite and halloysite 3% (v/v) and compost 1% (v/v) were mixed in with the soil. Soils without Ni, and amendments (0.0%) were designated as the control. Basal fertilization with a macro- and micronutrient fertilizer mixture (200 mg per pot) containing: N—26%, P2O5—12%, K2O—26%, B—0.013%, Cu—0.025%, Fe—0.05% and Mn—0.025% was applied to all pots. L. perenne (cv. Bokser) seeds, each weighing 5 g, were sown in the pots, germinating 6 days later. The plants were watered every other day with distilled water to 60% of the maximum water holding capacity of the soil by adding deionized water. The plants were harvested after 40 days and the soil was then collected. Plants were separated into shoots and roots. The rhizosphere soil tightly adhering to the roots was collected by brushing off, then air-dried and sifted through 2-mm sieves for determining Ni.
Phytotoxicity Study of Compost
The aim of the experiment carried out using the germination test and an early plant growth test Phytotoxkit™ [36] was to determine whether the addition of halloysite to soil polluted with Cu would have an influence on the development of the test plants. This test was based on measuring the germination and early growth ability of L. perenne roots in the analysed sample in comparison to the values obtained for a reference soil containing 85% sand, 10% kaolin, 5% peat, at pH 6.5–7.0 controlled with CaCO3. The test plates were placed vertically in a holder incubated at 25 °C for 3 days. At the end of incubation, a digital picture was captured for each of these test plates with the germinated plants. The assessment covered 25, 50, and 100% concentrations of compost from food waste. The 25, 50, and 100% concentrations of compost from food waste have been chosen on the basis of authors previous experiments [37, 38]. Measurements were taken in three repetitions for each amount of sorbent. The measurement of the length of the root for the Phytotoxkit test was carried out using Image Tool 3.0 software for Windows (UTHSCSA, San Antonio, TX, USA). The percentage of root growth inhibition (RI) was calculated with Formula (1)
where A is the mean root length in the control soil and B is the mean root length in the test.
Chemical Analysis of Plant Material
Before analysis, the plants were powdered using an analytical mill (Retsch type ZM300, Hann, Germany) and kept at ambient temperature in clean containers prior to chemical analyses. The roots and shoots were oven-dried at 55 °C to a stable weight and the dry biomass was recorded. A representative subsample (0.200 g) was digested in nitric acid (HNO3 p.a.) with a concentration of 1.40 g/cm and 30% H2O2 using a microwave oven (Milestone Start D, Italy). After filtration, the digestion products were adjusted to 100 mL volume with Milli-Q water (18.2 MΩ, Milli-Q Element A10 purification system, Millipore, Billerica, MA, USA). Extracts were analysed for HMs concentrations determined by the Atomic Absorption Spectrometry (AAS) method using an iCE-3000 spectrophotometer (Thermo Scientific, USA).
Laboratory equipment was acid-washed (20% HNO3) and rinsed with deionized water. All reagents were of analytical reagent grade unless otherwise stated. Each sample was processed in triplicate.
Soil Analytical Methods
The physicochemical properties of the soil were analysed before setting up the experiment as follows: soil pH with a glass electrode was analysed in a 1:5 soil/water suspension using a pH meter (Model pH/LF 12, Schott, Germany); organic matter content measured by Tiurin’s method after hot digestion of soil samples with K2Cr2O7 and H2SO4 in the presence of Ag2SO4 as a catalyst and the titration of K2Cr2O7 excess with FeSO4/(NH4)2SO4·6H2O [39]; hydrolytic acidity (HAC) by Kappen’s method, the soil samples were treated with 0.5 M/dm3 Ca-acetate solution adjusted to pH 8.2 in the ratio of 1:2.5 [40]; total exchangeable bases (TEB-K+, Na+,Ca2+, and Mg2+) by Kappen’s method through determining individual cations after extraction from the soil with CH3COONH4 [40]; cation exchange capacity (CEC) from the formula: CEC = HAC + TEB; percentage base saturation (V) from the formula: BS = 100.TEB/CEC−1; total N measured by the Kjeldahl method [41]; phosphorus (P) and potassium (K) content—Egner–Riehm method [42]; magnesium (Mg) content—atomic absorption spectrometry method following extraction using the Schachtschabel method [43].
Upon completing the experiment, for the determination of the trace element content in soil samples, 0.5 g of sieved soil were mineralized into a solution using a microwave oven (Milestone Start D, Italy) with a mixture of concentrated (69–71%) HNO3 and concentrated (37%) HCl (9:3 mL), in a partial digestion procedure equivalent to the USEPA Method 3051 A [44]. Certified reference material (Sigma Aldrich Chemie GmbH, No. SQC001, St. Louis, Missouri, MO, USA) was used for analyses. The exchangeable soil metal fractions were determined using 0.01 M CaCl2 (1:10 soil-extractant ratio) after agitation for 2 h at 20 °C. The extract was separated from the solid residue by centrifugation for 15 min [45].
Statistical Analysis
The data were analysed using Statistica software (version 10.0, San Diego, CA, USA). Significant differences (p < 0.05) between the mean values of different treatments were compared and evaluated using Duncan’s multiple range tests.
Results and Discussion
Phytotoxicity Evaluation of Compost
Ripe compost with no phytotoxins may be regarded as a source of nutrients improving physico-chemical and structural properties of the soil and accelerating germination [46]. Unripe composts with a high phytotoxicity value may lead to the inhibition of seed germination, destruction of roots, suppression of plant growth and damage to plants due to a high phytotoxin content [47]. Despite a wealth of study on composting, only few if any studies have attempted to determine toxicity of food waste compost. Mu et al. [48] conducted a test on plant-growth in order to examine how nutrients in compost from food waste were able to grow vegetables and what the quality was for vegetables grown with this compost. However, they did not provide the phytotoxicity test of the compost. An assessment of the phytotoxicity degree of compost from food waste is necessary to obtain high quality compost. At the end of a 3-day toxicity bioassay test (Fig. 1) using Phytotoxkit™, the root lengths of plants (L. perenne) for all compost concentrations, i.e. 25, 50, and 100%, were measured.
Figure 2 presents the lengths of the roots at a compost concentration of 25% for three replications (A–C). This concentration has a slightly stimulating effect on the root growth of the seeds (L. perenne) and the length of the roots at a concentration of 25% achieved the highest values (of all tested concentrations). The average length of the roots was 30.02 mm, while the average value for the longest root (of three replications) amounted to 42.33 mm.
Subsequently, samples with a compost concentration of 50% were subjected to an analysis. Figure 3 presents the lengths of the roots (L. perenne) at a compost concentration of 50% for all three replications. At a compost concentration of 50%, the lengths of the roots had much lower values compared to the length of the roots at a compost concentration of 25%. The average length of the roots was 14.38 mm and reached the average value for the longest root (26.67 mm).
The toxicity symptoms become severe with an increased dose of compost. Figure 4 presents the results and the average lengths of the roots at a compost concentration of 100%. Compared to the concentrations of 25 and 50%, it can be observed that this concentration had a strong inhibitory effect on plants. The average length of the roots was 0.98 mm.
Phytotoxicity is a measure of the delay or inhibition of seed germination, inhibition of plant growth or any adverse effect on plants caused by specific substances [49]. Thus, phytotoxicity bioassays can detect any substance capable of generating temporary or long-term stress on the germination capacity of seeds and roots growth [50]. For particular compost concentrations (25, 50, and 100%), the inhibition of the root growth was calculated. The results are presented in a graphical form in Fig. 5. A slightly stimulating effect on the growth of the roots can be noted at compost concentration of 25%, which amounted to—5.79%, while the other concentrations had an inhibitory effect on the growth of the roots and reached values of 49.33% for a compost concentration of 50%, and 92.45% for a compost concentration of 100%. The inhibitory effect on the growth of the roots increased with an increase in compost concentration.
Germination inhibitory substances are eliminated during composting. In general, the decrease in phytotoxicity during composting results from the degradation of phytotoxic substances by microorganisms [51]. The present study showed that the growth of the roots of L. perenne was slightly stimulated for a compost concentration of 25%, indicating the positive impact of this material on plant growth.
Biomass of L. perenne After Application of Compost with Sorbents
Since areas degraded and contaminated with heavy metals usually exhibit adverse conditions for the growth of plants, the application of various types of soil additives results in a stable and positive effect on plant growth and development [25]. The mineral and organic mixtures used in the experiment as additives supporting phytostabilization processes had a significant impact on the yield of above-ground parts of L. perenne. The toxic effect of Ni on plants is primarily largely determined by the plant species and its surplus is manifested by chlorosis (damage to the roots), which consequently limits the uptake and transport of nutrients to the above-ground parts of plants and the inhibition of photosynthesis and transpiration [52]. In the conducted experiment, no visible toxicity symptoms were observed in the test plant from the objects in which immobilizing additives had been applied. Plant biomass (dry mass) was measured at the end of the experiment and is shown in Fig. 6. The above-ground parts of the tested plant grown in soil without the addition of the amendments were characterized by high sensitivity to soil contamination with Ni. In the pot, to which the highest dose of Ni was applied (350 mg/kg of soil), a significant decrease (46%) in plant yield occurred compared to the control series. The authors’ own research [53,54,55] shows that increased Ni contents in a soil environment influence the reduction of the crop yield of plants, e.g. Triticum aestivum L., Halimione portulacoides, Zea mays L.
According to the results of the present study, for the greatest doses of Ni contamination (240 and 350 mg/kg), a mixture of compost and halloysite (+ 52%) turned out to be the most successful, resulting in increased crop yields of L. perenne compared to the control group (without immobilizing additives). These results indicate that the addition of the mineral and organic mixture stimulates plant growth and affects the phytostabilization process. In other experiment carried out by [25], the introduction of both chalcedonite as well as halloysite to soil, which had been contaminated with nickel caused an increase in the yield of the tested plants.
Effects of Amendments on Ni Concentration in L. perenne Organs
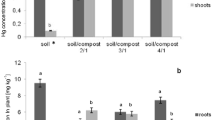
Nickel is an element with high mobility in the natural environment. The literature data confirm the correlation between the total amount of nickel in the soil and its concentration in plants [56]. This element is absorbed by hydrated aluminium and iron oxides, organic substances and clay materials, which contributes to its accumulation in the soil. The adverse effect of an excess Ni content of the soil on crop productivity and chemical composition of plants may be reduced by soil liming and fertilization with waste organic substances [57]. In the present study, the concentration of Ni in the roots and above-ground parts of L. perenne was closely correlated with the applied dose of Ni and mixtures of amendments (chalcedonite/compost, halloysite/compost) introduced into the soil. As shown in Fig. 7, the accumulation of Cr was much higher in roots than in shoots. In the control, Ni concentrations (in mg.kg−1 dry weight) varied between 5.94 and 12.49 in the shoots and between 12.49 and 205.42 in the roots.
It should be stressed that the uptake of metal from the soil into the roots is dependent on the bioavailability of the metal, as well as its mobility in the rhizosphere [58]. Compared with the control treatment, the mixture of compost from food waste and halloysite treatments significantly increased Ni concentration in plant roots as compared to pots in which additives had not been added. In another experiment conducted by Radziemska et al. [25, 29], the application of an immobilizing additive in the form of chalcedonite and halloysite caused significant increases in heavy metal concentrations in the roots of rye grass.
Effects of Amendments on Soil Chemical Properties
One of the main parameters affecting the successive course of phytostabilization processes is the maintenance of an optimum pH value in order to decrease the bioavailability of heavy metals. Both mineral and organic mixtures used in the experiment significantly increased the pH value of the soil (Fig. 8).
In the series lacking amendments, increasing doses of Ni contamination caused a successive increase in the soil pH. The greatest increase in pH was observed after compost with a chalcedonite mixture had been added to the soil. Generally, mineral amendment increases soil pH and favours the formation of oxides and metal carbonate precipitates, which decrease metal solubility [29]. In addition, plant roots can alter local soil pH through various rhizospheric processes such as assimilation, production of anions/cations and the release of organic acids [59]. Other authors [60,61,62] applied mineral sorbents to soils contaminated with heavy metals and reported a significant increase in the soil pH values. As regards the use of organic additives, similar results were obtained by Montiel-Rozas et al. [63], in which biosolid compost was used to immobilize heavy metals. This additive decreased the pH value of the soil by more than 1 U.
In order to immobilize heavy metals in soils, treatments based on the modification of the pH value of the soil as well as an increase in the sorption capacity of soils by the application of organic matter, e.g. composts, mineral sorbents or materials rich in clay fractions, are commonly applied. Since composts enhance the physical, physicochemical (including sorptive) and biological characteristics of soils, and are a significant source of nutrients, the authors used this material as an additive to the immobilizing mixture. In the experiment, the authors presented the possibilities for supporting the processes of Ni immobilization in the soil using a mineral and organic mixture comprising chalcedonite and halloysite as well as food waste compost. As regards heavy metal migration in the soil and water environment, it is not their total contents that are particularly important but the potentially mobile forms which may be available to plants and soil microorganisms, and/or may migrate down the soil profile to groundwater. The most important factors determining the mobility of heavy metals include the origin and chemical form, pH and sorption capacity, oxidation–reduction potential, and the formation of complex bonds with organic matter. In the presented research, the total and CaCl2-extractable Ni concentrations in soil depend on the dosage of soil contaminants and the type of amendments added (Fig. 9). Moreover, the concentration of CaCl2-extractable Ni in treatments with amendments was significantly lower than the total content. This suggests that soils treated with the application of the tested mineral and organic mixtures exhibit a greater ability to desorb Ni from the soil than soil without additives. In this study, the application of a mixture of compost from food waste and chalcedonite led to a significant decrease in total Ni concentrations in soil compared to the control pots.
In another experiment conducted by Radziemska and Mazur [64], the addition of mineral amendments to soil contaminated with HMs caused a significant decrease of Ni in the soil. The application of amendments reduced CaCl2-extractable content of Ni in soil, with the most significant reduction observed for mixture of compost from food waste and chalcedonite treatment. Compared with the control, treatment with this amendment reduced CaCl2-extractable Ni content by 61%. An analogical situation was observed when adding a mixture of compost from food waste and halloysite, although its influence was weaker. Alvarenga et al. [65] found that the application of both composts and liming materials led to a decrease in the level of mobile/effectively bioavailable fractions of heavy metals.
Conclusions
Composting FW in a vessel is a modern and necessary method for waste disposal and, at the same time, can be a solution to the problem of their excessive disposal in landfills. In recent years, an increasing emphasis has been placed on limiting the excessive inflow of FW and green waste to landfills, which certainly requires improved and accelerated methods enabling the transformation of these organic wastes into valuable products, such as e.g. soil amendment, which can be tackled through the proposed system. This study executed a pilot-scale experiment using an in-vessel composting system, which converts organic waste into good quality compost, which with mineral sorbents, can be used as a soil amendment. It was confirmed that a mixture of FW compost with mineral sorbents could be used to successfully phytostabilize Ni-contaminated soil. The biomass of L. perenne in particular organs depended on the dose of the Ni contaminant and the type of mineral and organic mixture incorporated into the soil. In the conducted experiment, Ni accumulated predominantly in the roots of L. perenne. The greatest increase in pH was observed after compost with the chalcedonite mixture was added to the soil. The application of mineral and organic mixture containing compost from FW and chalcedonite as a soil amendment tended to reduce the soil total and mobile fraction of Ni more than the un-amended soil.
References
Garcia-Garcia, G., Woolley, E., Rahimifard, S., Colwill, J., White, R., Needham, L.: A methodology for sustainable management of food waste. Waste Biomass Valori. 6, 2209–2227 (2017)
Pandey, P., Lejeune, M., Biswas, S., Morash, D., Weimer, B., Young, G.: A new method for converting food waste into pathogen free soil amendment for enhancing agricultural sustainability. J. Clean. Prod. 112, 205–213 (2016)
Pandey, P.K., Vaddella, V., Cao, W., Biswas, S., Chiu, C., Hunter, S.: In-vessel composting system for converting food and green wastes into pathogen free soil amendment for sustainable agriculture. J. Clean. Prod. 139, 407–415 (2016)
Joo, H.S., Shoda, M., Phae, C.G.: Degradation of diesel oil in soil using a food waste composting process. Biodegradation 18, 597 (2007)
Loizidou, M., Alamanou, D.G., Sotiropoulos, A., Lytras, C., Mamma, D., Malamis, D., Kekos, D.: Pilot scale system of two horizontal rotating bioreactors for bioethanol production from household food waste at high solid concentrations. Waste Biomass Valori. 5, 1709–1719 (2017)
Watteau, F., Villemin, G.: Characterization of organic matter microstructure dynamics during co-composting of sewage sludge, barks and green waste. Bioresour. Technol. 102, 9313–9317 (2011)
Zhou, Y., Selvam, A., Wong, J.W.C.: Evaluation of humic substances during co-composting of food waste, sawdust and Chinese medicinal herbal residues. Bioresour. Technol. 168, 229–234 (2014)
Jara-Samaniego, J., Pérez-Murcia, M.D., Bustamante, M.A., Pérez-Espinosa, A., Paredes, C., López, M., Moral, R.: Composting as sustainable strategy for municipal solid waste management in the Chimborazo Region, Ecuador: suitability of the obtained composts for seedling production. J. Clean. Prod. 141, 1349–1358 (2017)
Voběrková, S., Vaverková, M.D., Burešová, A., Adamcová, D., Vršanská, M., Kynický, J., Brtnický, M., Adam, V.: Effect of inoculation with white-rot fungi and fungal consortium on the composting efficiency of municipal solid waste. Waste Manag. 61, 157–164 (2017)
Barrena, R., Turet, J., Busquets, A., Farrés, M., Font, X., Sánchez, A.: Respirometric screening of several types of manure and mixtures intended for composting. Bioresour. Technol. 102, 1367–1377 (2011)
Wang, X., Selvam, A., Chan, M., Wong, J.W.C.: Nitrogen conservation and acidity control during food wastes composting through struvite formation. Bioresour. Technol. 147, 17–22 (2013)
Jiang, T., Ma, X., Yang, J., Tang, Q., Yi, Z., Chen, M., Li, G.: Effect of different struvite crystallization methods on gaseous emission and the comprehensive comparison during the composting. Bioresour. Technol. 217, 219–226 (2016)
Kim, J.K., Lee, D.J., Ravindran, B., Jeong, K.-H., Wong, J.W.-C., Selvam, A., Karthikeyan, O.P., Kwag, J.-H.: Evaluation of integrated ammonia recovery technology and nutrient status with an in-vessel composting process for swine manure. Bioresour. Technol. 245, 365–371 (2017)
Barral, S.M.T., Menduíña, A.M., Cendón, S.Y., Viqueira, D.F.: Assessment of municipal solid waste compost quality using standardized methods before preparation of plant growth media. Waste Manag. Res. 25, 99–108 (2007)
Shi, H., Wang, X.C., L.,i Q., Jiang: S.: Degradation of typical antibiotics during human feces aerobic composting under different temperatures. Environ. Sci. Pollut. Res. 23, 1–12 (2016)
Waqas, M., Nizami, A.S., Aburiazaiza, A.S., Barakat, M.A., Ismail, I.M.I., Rashid, M.I.: Optimization of food waste compost with the use of biochar. J. Environ. Manag. (2017). https://doi.org/10.1016/j.jenvman.2017.06.015
Adamcová, D., Vaverková, M.D., Bartoň, S., Havlícek, Z., Broušková, E.: Soil contamination in landfills: a case study of a landfill in Czech Republic. Solid Earth 7, 239–247 (2016)
Vaverková, M.D., Adamcová, D.: Heavy metals uptake by select plant species in the landfill area of tepanovice, Czech Republic. Pol. J. Environ. Stud. 23, 2265–2269 (2014)
Vaverková, M.D., Zloch, J., Adamcová, D., Radziemska, M., Vyhnánek, T., Trojan, V., Winkler, J., Đorđević, B., Elbl, J., Brtnický, M.: Landfill leachate effects on germination and seedling growth of hemp cultivars (Cannabis sativa L.). Waste Biomass Valori. (2017). https://doi.org/10.1007/s12649-017-0058-z
Adamcová, D., Radziemska, M., Ridošková, A., Bartoň, S., Pelcová, P., Elbl, J., Kynický, J., Brtnický, M., Vaverková, M.D.: Environmental assessment of the effects of a municipal landfill on the content and distribution of heavy metals in Tanacetum vulgare L. Chemosphere 185, 1011–1018 (2017)
Dai, S., Li, Y., Zhou, T., Zhao, Y.: Reclamation of heavy metals from contaminated soil using organic acid liquid generated from food waste: removal of Cd, Cu, and Zn, and soil fertility improvement. Environ. Sci. Pollut. Res. 18, 15260–15269 (2017)
Liu, C.C., Lin, Y.C.: Reclamation of copper-contaminated soil using EDTA or citric acid coupled with dissolved organic matter solution extracted from distillery sludge. Environ Pollut. 178, 97–101 (2013)
Ghosh, M., Singh, S.P.: A review on phytoremediation of heavy metals and utilization of its byproducts. Appl. Ecol. Environ. Res. 3, 1–18 (2005)
Santibáñez, C., Verdugo, C., Ginocchio, R.: Phytostabilization of copper mine tailings with biosolids: Implications for metal uptake and productivity of Lolium perenne. Sci. Total Environ. 395, 1–10 (2008)
Radziemska, M., Gusiatin, M., Bilgin, A.: Potential of using immobilizing agents in aided phytostabilization on simulated contamination of soil with lead. Ecol. Eng. 102, 490–500 (2017)
Smith, R.A.H., Bradshaw, A.D.: The use of metal tolerant plant populations for the reclamation of metalliferous wastes. J. Appl. Ecol. 16, 595–612 (1979)
Pichtel, J., Salt, C.A.: Vegetative growth and trace metal accumulation on metalliferous wastes. J. Environ. Qual. 27, 618–624 (1998)
Arienzo, M., Adamo, P., Cozzolino, V.: The potential of Lolium perenne for revegetation of contaminated soil from a metallurgical site. Sci. Total Environ. 319, 13–25 (2004)
Radziemska, M., Vaverková, M.D., Baryła, A.: Phytostabilization—management strategy for stabilizing trace elements in contaminated soils. Int. J. Environ. Res. Public Health 14, 958 (2017)
Kumpiene, J., Mench, M., Bes, C.M., Fitts, J.P.: Assessment of aided phytostabilization of copper-contaminated soil by X-ray absorption spectroscopy and chemical extractions. Environ. Pollut. 159, 1536–1542 (2011)
Li, M., Mohamed, I., Raleve, D., Chen, W., Huang, Q.: Field evaluation of intensive compost application on Cd fractionation and phytoavailability in a mining-contaminated soil. Environ. Geochem. Health 38, 1193–1201 (2015)
Jones, S., Bardos, R.P., Kidd, P.S., Mench, M., Leij, F., Hutchings, T., Cundy, A., Joyce, C., Soja, G., Friesl-Hanl, W., Herzig, R., Menger, P.: Biochar and compost amendments enhance copper immobilization and support plant growth in contaminated soils. J. Environ. Manag. 171, 101–112 (2016)
Yang, T.T., Liu, J., Chen, W.C., Chen, X., Shu, H.Y., Jia, P., Liao, B., Shu, W.S., Li, J.T.: Changes in microbial community composition following phytostabilization of an extremely acidic Cu mine tailings. Soil Biol. Biochem. 114, 52–58 (2017)
Zhang, H., Li, G., Gu, J., Wang, G., Li, Y., Zhang, D.: Influence of aeration on volatile sulfur compounds (VSCs) and NH3 emissions during aerobic composting of kitchen waste. Waste Manag. 58, 369–375 (2016)
Yang, F., Li, G., Shi, H., Wang, Y.: Effects of phosphogypsum and superphosphate on compost maturity and gaseous emissions during kitchen waste composting. Waste Manag. 36, 70–76 (2015)
Phytotoxkit: Seed germination and early growth microbiotest with higher plants. In: Standard Operation Procedure, pp. 1–24. MicroBioTests Inc., Nazareth (2004)
Adamcová, D., Vaverková, M.D., Břousková, E.: The toxicity of two types of sewage sludge form wastewater treatment plant for plants. J. Ecol. Eng. 17, 33–37 (2016)
Křivanková, E., Adamcová, D., Vaverková, M.D., Havlíček, Z.: Use of Phytotoxkit (TM) test in assessment of toxicity of two types of sewage sludge. Proceedings of International PHD Students Conference, (MENDELNET), pp. 435–440 (2016)
Radziemska, M., Vaverková, M.D., Baryła., A.: Phytostabilization—management strategy for stabilizing trace elements in contaminated soils. Int. J. Environ. Res. Public Health 14, 9 (2017)
Mocek, A., Drzymała, S.: Genesis, analysis and soil classification, p. 418. Poznan University of Life Sciences (in Polish) (2010)
Klute, A.: Methods of soil analysis. American Society of Agronomy. Agronomy Monograph, Madison (1996)
Black, G.R.: Bulk density: method of soil analysis. Monograph no. 9 part I. American Society of Agronomy Inc, Washington, DC (1965)
Riehm, H.: Die ammoniumlaktatessigsaure-methode zur bestimmung derleichtloeslichen phosphosaure in karbonathaltigen boden. Agrochimica. 3, 49–65 (1958)
Lityński, T., Jurkowska, H., Gorlach, E.: Chemical and Agriculture Analysis, pp. 129–132. PWN, Warszawa (1976) (Polish)
U.S. Environmental Protection Agency, Microwave assisted acid digestion of sediments, sludge’s, soils, and oils. Office of Solid Waste and Emergency Response, U.S. Government Printing Office, Washington, DC (1998)
Pueyo, M., López-Sanchez, J.F., Rauret, G.: Assessment of CaCl2, NaNO3 and NH4NO3 extraction procedures for the study of Cd, Cu, Pb and Zn extractability in contaminated soils. Anal. Chim. Acta 504, 217–226 (2004)
He, X.S., Xi, B.D., Zhang, Z.Y., Gao, R.T., Tan, W.B., Cui, D.Y., Yuan, Y.: Composition removal, redox, and metal complexation properties of dissolved organic nitrogen in composting leachates. J. Hazard. Mater. 283, 227–233 (2015)
Mu, D., Horowitz, N., Casey, M., Jones, K.: Environmental and economic analysis of an in-vessel food waste composting system at Kean University in the U.S. Waste Manag. 59, 476–486 (2017)
Cui, H.-Y., Zhao, Y., Chen, Y.N., Zhang, X., Wang, X.Q., Lu, Q., Jia, L.M., Wei, Z.M.: Assessment of phytotoxicity grade during composting based on EEM/PARAFAC combined with projection pursuit regression. J. Hazard. Mater. 326, 10–17 (2017)
Pinho, I.A., Lopes, D.V., Martins, R.C., Quina, M.J.: Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 185, 258–267 (2017)
Pavel, L.V., Sobatiu, D.L., Diaconu, M., Statescu, F., Gavrilescu, M.: Effects of heavy metals on Lepidium sativum germination and growth Environ. Eng. Manag. J. 12, 727–733 (2013)
Aparnaa, C., Sarithaa, P., Himabindua, V., Anjaneyulu, Y.: Techniques for the evaluation of maturity for composts of industrially contaminated lake sediments. Waste Manag. 28(10), 1773–1784 (2008)
Rizwan, M., Mustofa, M.G., Ahmad, M.Z., Imtiaz, M., Mehmood, S., Adeel, M., Dai, Z., Li, Z., Aziz, O., Zhang, Y., Tu, S.: Nitric oxide induces rice tolerance to excessive nickel by regulating nickel uptake, reactive oxygen species detoxification and defense-related gene expression. Chemosphere ISSN 0045-6535 (2007)
Gajewska, E., Sklodowska, M., Słaba, M., Mazur, J.: Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Plant Biol. 50, 653–659 (2006)
Duatre, B., Delgado, M., Caador, I.: The role of citric acid in cadmium and nickel uptake and translocation, in Halimione portulacoides. Chemosphere 69, 836–840 (2007)
Narwal, R.P., Singh, M., Gupta, A.P., Khusad, M.S.: Nickel and Zn interaction in corn grown on sewer irrigated soil. Crop Res. 7, 366–372 (1994)
Kabata-Pendias, A.: Trace Elements in Soils and Plants, 4th edn. CRC Press, Boca Raton (2011)
Kalembasa, S., Kuziemska, B.: The effect of soil contamination with nickel on the yield of Dactylis glomerata). Zesz. Probl. Post Nauk Rol. 512, 297304 (2006)
Maestri, E., Marmiroli, M.: Genetic and molecular aspects of metal tolerance and hyperaccumulation. In: Gupta, D.K., Sandalio, L.M. (eds.) Metal Toxicity in Plants: Perception, Signalling and Remediation, pp. 41–63. Springer, Berlin (2012)
Bolan, S.N., Park, J.H., Robinson, B., Naidu, R., Huh, K.Y.: Phytostabilization: a green approach to contaminant containment. Adv. Agron. 112, 145–204 (2011)
Ye, X., Kang, S., Wang, H., Li, H., Zhang, Y., Wang, G., Zhaoa, Y.: Modified natural diatomite and its enhanced immobilization of lead, copper and cadmium in simulated contaminated soils. J. Hazard. Mater. 289, 210–218 (2015)
Jin, Y., Liu, W., Li, H.-L., Shen, S.-G., Liang, S.-X., Liu, C., Shan, L.: Nano-hydroxyapatite immobilized lead and enhanced plant growth of ryegrass in a contaminated soil. Ecol. Eng. 95, 25–29 (2016)
Montiel-Rozas, M.M., Madejón, E., Madejón, P.: Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous species: An assessment in sand and soil conditions under different levels of contamination. Environ. Pollut. 216, 273–281 (2016)
Radziemska, M., Mazur, Z.: Content of selected heavy metals in Ni-contaminated soil following the application of halloysite and zeolite. J. Ecol. Eng. 17(3), 125–133 (2016)
Alvarenga, P., Gonçalves, A.P., Fernandes, R.M., de Varennes, A., Vallini, G., Duarte, E., Cunha-Queda, A.C.: Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci. Tot. Environ. 406, 43–56 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Radziemska, M., Vaverková, M.D. & Mazur, Z. Pilot Scale Use of Compost Combined with Sorbents to Phytostabilize Ni-Contaminated Soil Using Lolium perenne L.. Waste Biomass Valor 10, 1585–1595 (2019). https://doi.org/10.1007/s12649-017-0166-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0166-9