Abstract
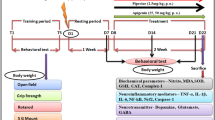
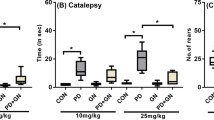
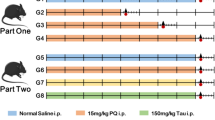
Janus-activated kinases (JAKs) are well known to play a physiological as well as pathological role in several disease conditions such as autoimmune disorders. The present study evaluated the therapeutic potential of CP690550 (pan-JAK inhibitor) in 1-methyl-4-phenyl-1,2,3,6-tertahydropyridine (MPTP) model of Parkinson’s disease. Intrastriatal administration of MPTP (30 micromol in 2 microl) produced a significant alteration in behavioural (bar test and block test). Biochemical investigations in serum and brain homogenate revealed a significant alteration in the JAK-mediated cytokine levels. MPTP administration also showed significant imbalance of inflammatory (increased TNF-α, IL-6 and NF-κb) versus anti-inflammatory cytokines (decreased IL-10 levels). MPTP-treated brain sections revealed alteration in the tissue architecture as well as undifferentiated bodies of varying contour and lesions. Chronic administration of CP690550 (3 and 10 mg/kg, po) for 7 days significantly reversed the behavioural, biochemical and histological alterations induced by MPTP. In conclusion, the findings of the present study govern the possible therapeutic potential of CP690550 in MPTP-treated mice and thus highlight the therapeutic potential of JAK inhibitors in treatment of Parkinson’s disease.








Similar content being viewed by others
References
Agid YJTL (1991) Parkinson’s Disease: Pathophysiology 337:1321–1324
Aguirre JA, Leo G, Cueto R, Andbjer B, Naylor A, Medhurst AD, Agnati LF, Fuxe K (2008) The novel cyclooxygenase-2 inhibitor GW637185X protects against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine toxicity. Neuroreport 19:657–660
Appel SH, Beers DR, Henkel JS (2010) T cell-microglial dialogue in Parkinson's disease and amyotrophic lateral sclerosis: are we listening?. Trends Immunol 31(1):7–17
Bartels AL, Leenders KL (2007) Neuroinflammation in the pathophysiology of Parkinson’s disease: evidence from animal models to human in vivo studies with [11C]‐PK11195 PET 22:1852–1856
Benetti F (2012) Gustincich S and Legname GJEoodd. Gene Expression Profiling and Therapeutic Interventions in Neurodegenerative Diseases: a Comprehensive Study on Potentiality and Limits 7:245–259
Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem 73(3):1127–1137
Cao S, Theodore S, Standaert DG (2010) Fcγ receptors are required for NF-κB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson's disease. Mol neurodegener 5(1):1–2
Chakrabarty P, Ceballos-Diaz C, Lin WL, Beccard A, Jansen-West K, McFarland NR, Janus C, Dickson D, Das P, Golde TE (2011) Interferon-γ Induces Progressive Nigrostriatal Degeneration and Basal Ganglia Calcification 14:694–696
Chen H, O'Reilly EJ, Schwarzschild MA, Ascherio A (2008) Peripheral Inflammatory Biomarkers and Risk of Parkinson’s Disease 167:90–95
Chung YC, Kim SR, Park JY, Chung ES, Park KW, Won SY, Bok E, Jin M, Park ES, Yoon SH (2011) Fluoxetine Prevents MPTP-Induced Loss of Dopaminergic Neurons by Inhibiting Microglial Activation 60:963–974
Dong Y, Benveniste EN (2001) Immune Function of Astrocytes 36:180–190
Dowty ME, Lin J, Ryder TF, Wang W, Walker GS, Vaz A, Chan GL, Krishnaswami S, Prakash C (2014) The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a janus kinase inhibitor, in humans. Drug Metab Dispos 42(4):759–773
Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N (2013) Toll‐like receptor 4 is required for α‐synuclein dependent activation of microglia and astroglia. Glia 61(3):349–360
Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, Ladner KJ, Bevan AK, Foust KD, Godbout JPJN (2014) Microglia Induce Motor Neuron Death via the Classical NF-Κb Pathway in Amyotrophic Lateral Sclerosis 81:1009–1023
Funk N, Wieghofer P, Grimm S, Schaefer R, Bühring HJ, Gasser T, Biskup SJMD (2013) Characterization of Peripheral Hematopoietic Stem Cells and Monocytes in Parkinson’s Disease 28:392–395
Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B (2002) Microglial Activation-Mediated Delayed and Progressive Degeneration of Rat Nigral Dopaminergic Neurons: Relevance to Parkinson’s Disease 81:1285–1297
Ghoreschi K, Laurence A, O’Shea JJ (2009) Janus Kinases in Immune Cell Signaling 228:273–287
Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K (2007) Selective Inhibition of NF-Κb Activation Prevents Dopaminergic Neuronal Loss in a Mouse Model of Parkinson’s Disease 104:18754–18759
Gray GK, McFarland BC, Nozell SE, Benveniste EN (2014) NF-κB and STAT3 in glioblastoma: therapeutic targets coming of age. Expert Rev Neurother 14(11):1293–1306
Gupta A, Kumar A, Kulkarni SK (2010) Licofelone attenuates MPTP-induced neuronal toxicity: behavioral, biochemical and cellular evidence. Inflammopharmacology 18(5):223–232
Hirsch E, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PJDP (2003) The Role of Dial Reaction and Inflammation in Parkinson’s Disease 991:214–228
Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G (2007) Inflammation Induces Mitochondrial Dysfunction and Dopaminergic Neurodegeneration in the Nigrostriatal System 100:1375–1386
Kalonia H, Kumar P, Kumar A, Nehru B (2009) Effect of caffeic acid and rofecoxib and their combination against intrastriatal quinolinic acid induced oxidative damage, mitochondrial and histological alterations in rats. Inflammopharmacology 17(4):211–219
Kannarkat GT, Boss JM, Tansey MG (2013) The Role of Innate and Adaptive Immunity in Parkinson’s Disease 3:493–514
Kim YS, Joh TH (2006) Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med 38(4):333–347
Migita K, Miyashita T, Izumi Y, Koga T, Komori A, Maeda Y, Jiuchi Y, Aiba Y, Yamasaki S, Kawakami A, Nakamura M (2011) Inhibitory effects of the JAK inhibitor CP690, 550 on human CD4+ T lymphocyte cytokine production. BMC immunol 12(1):1–0
O'Shea JJ, Murray PJ (2008) Cytokine Signaling Modules in Inflammatory Responses 28:477–487
O’Shea JJ, Schwartz DM, Villarino AV, Gadina M (2015) McInnes IB and Laurence AJArom. The JAK-STAT Pathway: Impact on Human Disease and Therapeutic Intervention 66:311–328
O'Sullivan LA, Liongue C, Lewis RS, Stephenson SE, Ward AC (2007) Cytokine receptor signaling through the Jak–Stat–Socs pathway in disease. Mol immunol 44(10):2497–506
O’shea JJ (2004) Targeting the Jak/STAT pathway for immunosuppression. Annals of the rheumatic diseases 63(suppl 2):ii67–71
Pattison MJ, MacKenzie KF, Arthur JS (2012) Inhibition of JAKs in macrophages increases lipopolysaccharide-induced cytokine production by blocking IL-10–mediated feedback. The J Immunol 189(6):2784–2792
Shuai K, Liu B (2003) Regulation of JAK–STAT signalling in the immune system. Nat Rev Immunol 3(11):900–911
Strober B, Buonanno M, Clark JD, Kawabata T, Tan H, Wolk R, Valdez H, Langley RG, Harness J, Menter A, Papp K (2013) Effect of tofacitinib, a J anus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol 169(5):992–999
Yarilina A, Xu K, Chan C, Ivashkiv LB (2012) Regulation of inflammatory responses in tumor necrosis factor–activated and rheumatoid arthritis synovial macrophages by JAK inhibitors. Arthritis & Rheumatism 64(12):3856–3866
Yasuda T, Nakata Y, Mochizuki H (2003) α-Synuclein and neuronal cell death. Mol Neurobiol 47(2):466–483
Acknowledgements
The authors are thankful to the researchers supporting project number (RSP2022R491), King Saud University, Riyad, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alshammari, A., Alharbi, M., Albekairi, N.A. et al. Protective Effect of CP690550 in MPTP-Induced Parkinson’s Like Behavioural, Biochemical and Histological Alterations in Mice. Neurotox Res 40, 564–572 (2022). https://doi.org/10.1007/s12640-022-00498-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-022-00498-3