Abstract
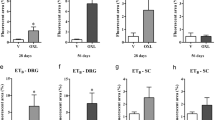
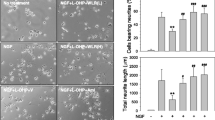
Oxaliplatin-induced neurotoxicity is expressed as a dose-limiting peripheral sensory neuropathy (PSN). Cannabinoid substances have been investigated for the analgesic effect. This study aimed to investigate the role of cannabinoid receptors in oxaliplatin-associated PSN. Swiss male mice received nine oxaliplatin injections (2 mg/kg, i.v.). Mechanical and thermal nociceptive tests were performed for 56 days. CB1, CB2, and c-Fos expression were assessed in dorsal root ganglia (DRG), spinal cord (SC), trigeminal ganglia (TG), spinal trigeminal nucleus caudalis (Sp5C), and periaqueductal gray (PAG). Iba-1 expression was assessed in DRG and ATF3 in TG. Cannabidiol (10 mg/kg, p.o.) or a CB1/CB2 non-selective agonist (WIN 55,212–2; 0.5 mg/kg, s.c.) or AM251 (CB1 antagonist) or AM630 (CB2 antagonist) (3 mg/kg, i.p.) were injected before oxaliplatin. Oxaliplatin increased CB1 in DRG, SC, TG, Sp5C, and ventrolateral PAG, with no interference in CB2 expression. Cannabidiol increased CB1 in DRG, reduced mechanical hyperalgesia and c-Fos expression in DRG and SC. Additionally, WIN 55,212–2 increased CB1 in DRG, reduced mechanical hyperalgesia, cold allodynia and c-Fos expression in DRG and SC. CB1 blockage hastened the cold allodynia response, but the CB2 antagonist failed to modulate the oxaliplatin-induced nociceptive behavior. Oxaliplatin also increased Iba-1 in DRG, suggesting immune response modulation which was reduced by cannabidiol and enhanced by AM630. The modulation of the endocannabinoid system, through the CB1 receptor, attenuates the oxaliplatin-associated PNS. The activation of the endocannabinoid system could be considered as a therapeutic target for controlling oxaliplatin-associated neuropathy.










Similar content being viewed by others
Abbreviations
- 2-AG:
-
2-Arachidonoylglycerol
- AEA:
-
Anandamide
- AM251:
-
Cannabinoid CB1 receptor antagonist
- AM630:
-
Cannabinoid CB2 receptor antagonist
- ATF3:
-
Activating transcription factor 3
- CB1:
-
Cannabinoid CB1 receptor
- CB2:
-
Cannabinoid CB2 receptor
- CBD:
-
Cannabidiol
- DRG:
-
Dorsal root ganglia
- Iba-1:
-
Ionized calcium-binding adapter molecule 1
- iNOS:
-
Inducible nitric oxide synthase
- OXL:
-
Oxaliplatin
- PAG:
-
Periaqueductal gray
- PFA:
-
Paraformaldehyde
- PSN:
-
Peripheral sensory neuropathy
- SC:
-
Spinal cord
- Sp5C:
-
Spinal trigeminal nucleus caudalis
- TG:
-
Trigeminal ganglia
- TRPV1:
-
Transient receptor potential vanilloid 1
- vlPAG:
-
Ventrolateral periaqueductal gray
- WIN:
-
WIN 55, 212–2
References
Argyriou AA (2015) Updates on oxaliplatin-induced peripheral neurotoxicity (OXAIPN). Toxics 3:187–197. https://doi.org/10.3390/toxics3020187
Argyriou AA, Bruna J, Marmiroli P, Cavaletti G (2012) Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol 82:51–77. https://doi.org/10.1016/j.critrevonc.2011.04.012
Branca JJV, Maresca M, Morucci G, Becatti M, Paternostro F, Gulisano M et al. (2018) Oxaliplatin-induced blood brain barrier loosening: a new point of view on chemotherapy-induced neurotoxicity. Oncotarget 9:23426–23438. https://doi.org/10.18632/oncotarget.25193
Calls A, Carozzi V, Navarro X, Monza L, Bruna J (2020) Pathogenesis of platinum-induced peripheral neurotoxicity: Insights from preclinical studies. Exp Neurol. https://doi.org/10.1016/j.expneurol.2019.113141
Cavaletti G, Marmiroli P (2020) Management of oxaliplatin-induced peripheral sensory neuropathy. Cancers (basel) 12:1370. https://doi.org/10.3390/cancers12061370
Chiocchetti R, Galiazzo G, Tagliavia C, Stanzani A, Giancola F, Menchetti M et al (2019) Cellular distribution of canonical and putative cannabinoid receptors in canine cervical dorsal root ganglia. Front Vet Sci 6:313. https://doi.org/10.3389/fvets.2019.00313
Cremolini C, Schirripa M, Antoniotti C, Moretto R, Salvatore L, Masi G et al (2015) First-line chemotherapy for mCRC-a review and evidence-based algorithm. Nat Rev Clin Oncol 12:607–619. https://doi.org/10.1038/nrclinonc.2015.129
Cunha TM, Verri WA Jr, Vivancos GG, Moreira IF, Reis S, Parada CA et al (2004) An electronic pressure-meter nociception paw test for mice. Brazilian J Med Biol Res 37:401–407. https://doi.org/10.1590/S0100-879X2004000300018
Di Cesare Mannelli L, Pacini A, Micheli L, Tani A, Zanardelli M, Ghelardini C (2014) Glial role in oxaliplatin-induced neuropathic pain. Exp Neurol 261:22–33. https://doi.org/10.1016/j.expneurol.2014.06.016
Drott J, Fomichov V, Starkhammar H, Börjeson S, Kjellgren K, Berterö C (2019) Oxaliplatin-induced neurotoxic side effects and their impact on daily activities: a longitudinal study among patients with colorectal cancer. Cancer Nurs 42:E40–E48. https://doi.org/10.1097/ncc.0000000000000674
Dunham NW, Miya TS (1957) A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim) 46:208–209. https://doi.org/10.1002/jps.3030460322
Eddy NB, Leimbach D (1953) Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther 107:385–393
Gobira PH, Vilela LR, Gonçalves BDC, Santos RPM, de Oliveira AC, Vieira LB et al (2015) Cannabidiol, a Cannabis sativa constituent, inhibits cocaine-induced seizures in mice: possible role of the mTOR pathway and reduction in glutamate release. Neurotoxicology 50:116–121. https://doi.org/10.1016/j.neuro.2015.08.007
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK et al (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23–30. https://doi.org/10.1200/JCO.2004.09.046
Gondim DV, Araújo JCB, Cavalcante ALC, Havt A, da Siva Quetz J, de Castro Brito GA et al (2012) CB1 and CB2 contribute to antinociceptive and anti-inflammatory effects of electroacupuncture on experimental arthritis of the rat temporomandibular joint. Can J Physiol Pharmacol 90:1479–1489. https://doi.org/10.1139/y2012-130
Gondinho PDAR, de Barros Silva PG, Lisboa MRP, Costa BA, da Rocha Filho DR, Gifoni MAC et al (2020) FLOX (5-fluorouracil + leucovorin + oxaliplatin) chemotherapy for colorectal cancer leads to long-term orofacial neurotoxicity: a STROBE-guided longitudinal prospective study. Int J Clin Oncol 25(12):2066–2074. https://doi.org/10.1007/s10147-020-01757-z
Guindon J, Hohmann AG (2009) The endocannabinoid system and pain. CNS Neurol Disord Drug Targets 8:403–421. https://doi.org/10.2174/187152709789824660
Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG (2013) Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol Res 67:94–109. https://doi.org/10.1016/j.phrs.2012.10.013
Hald A, Ding M, Egerod K, Hansen RR, Konradsen D, Jørgensen SG et al (2008) Differential effects of repeated low dose treatment with the cannabinoid agonist WIN 55,212–2 in experimental models of bone cancer pain and neuropathic pain. Pharmacol Biochem Behav 91:38–46. https://doi.org/10.1016/j.pbb.2008.04.021
Harris HM, Sufka KJ, Gul W, Elsohly MA (2016) Effects of delta-9-tetrahydrocannabinol and cannabidiol on cisplatin-induced neuropathy in mice. Planta Med 82:1169–1172. https://doi.org/10.1055/s-0042-106303
Heinricher MM, Tavares I, Leith JL, Lumb BM (2009) Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev 60:214–225. https://doi.org/10.1016/j.brainresrev.2008.12.009
Howlett AC, Abood ME (2017) CB1 and CB2 receptor pharmacology. Adv Pharmacol 80:169–206. https://doi.org/10.1016/bs.apha.2017.03.007
Kamimura R, Hossain MZ, Unno S, Ando H, Masuda Y, Takahashi K et al (2018) Inhibition of 2-arachydonoylgycerol degradation attenuates orofacial neuropathic pain in trigeminal nerve-injured mice. J Oral Sci 60:37–44. https://doi.org/10.2334/josnusd.17-0005
King KM, Myers AM, Soroka-Monzo AJ, Tuma RF, Tallarida RJ, Walker EA et al (2017) Single and combined effects of Δ9-tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br J Pharmacol 174:2832–2841. https://doi.org/10.1111/bph.13887
Leonard GD, Wright MA, Quinn MG, Fioravanti S, Harold N, Schuler B et al (2005) Survey of oxaliplatin-associated neurotoxicity using an interview-based questionnaire in patients with metastatic colorectal cancer. BMC Cancer 5:116. https://doi.org/10.1186/1471-2407-5-116
Li D, Kim W, Shin D, Jung Y, Bae H, Kim SK (2016) Preventive effects of bee venom derived phospholipase A2 on oxaliplatin-induced neuropathic pain in mice. Toxins 8:27. https://doi.org/10.3390/toxins8010027
Liang YC, Huang CC, Sen HK (2007) The synthetic cannabinoids attenuate allodynia and hyperalgesia in a rat model of trigeminal neuropathic pain. Neuropharmacology 53:169–177. https://doi.org/10.1016/j.neuropharm.2007.04.019
Ling J, Erol F, Viatchenko-Karpinski V, Kanda H, Gu JG (2017) Orofacial neuropathic pain induced by oxaliplatin: downregulation of KCNQ2 channels in V2 trigeminal ganglion neurons and treatment by the KCNQ2 channel potentiator retigabine. Mol Pain 13:1744806917724715. https://doi.org/10.1177/1744806917724715
Maldonado R, Baños JE, Cabañero D (2016) The endocannabinoid system and neuropathic pain. Pain 157:S23–S32. https://doi.org/10.1097/j.pain.0000000000000428
McDonough P, McKenna JP, McCreary C, Downer EJ (2014) Neuropathic orofacial pain: cannabinoids as a therapeutic avenue. Int J Biochem Cell Biol 55:72–78. https://doi.org/10.1016/j.biocel.2014.08.007
Metna-Laurent M, Mondésir M, Grel A, Vallée M, Piazza PV (2017) Cannabinoid-induced tetrad in mice. Curr Protoc Neurosci 80:9–59. https://doi.org/10.1002/cpns.31
Necker R, Hellon RF (1978) Noxious thermal input from the rat tail: modulation by descending inhibitory influences. Pain 4:231–242. https://doi.org/10.1016/0304-3959(77)90135-X
Ossato A, Canazza I, Trapella C, Vincenzi F, De LMA, Rimondo C et al (2016) Effect of JWH-250, JWH-073 and their interaction on ‘tetrad’, sensorimotor, neurological and neurochemical responses in mice. Prog Neuro-Psychopharmacology Biol Psychiatry 67:31–50. https://doi.org/10.1016/j.pnpbp.2016.01.007
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M et al (2020) The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br J Pharmacol 177:3617–3624. https://doi.org/10.1111/bph.15193
Pereira AF, de Oliveira FFB, de Freitas Alves BW, de Menezes KLS, de Mesquita AKV, Lisboa MRP et al (2018) Neurotoxic effect of oxaliplatin: comparison with its oxalate-free analogue cis-[PtII(1R,2R-DACH)(3-acetoxy-1,1-cyclobutanedicarboxylato)] (LLC-1402) in mice. Toxicol Appl Pharmacol 340:77–84. https://doi.org/10.1016/j.taap.2018.01.001
Samineni VK, Grajales-Reyes JG, Copits BA, O’Brien DE, Trigg SL, Gomez AM et al. (2017) Divergent modulation of nociception by glutamatergic and GABAergic neuronal subpopulations in the periaqueductal gray. ENeuro 4(2). https://doi.org/10.1523/ENEURO.0129-16.2017
Sanberg PR, Bunsey MD, Giordano M, Norman AB (1988) The catalepsy test: its ups and downs. Behav Neurosci 102:748–759. https://doi.org/10.1037//0735-7044.102.5.748
Shiue SJ, Peng HY, Lin CR, Wang SW, Rau RH, Cheng JK (2017) Continuous intrathecal infusion of cannabinoid receptor agonists attenuates nerve ligation-induced pain in rats. Reg Anesth Pain Med 42:499–506. https://doi.org/10.1097/AAP.0000000000000601
Silva NR, Gomes FV, Fonseca MD, Mechoulam R, Breuer A, Cunha TM et al (2017) Antinociceptive effects of HUF-101, a fluorinated cannabidiol derivative. Prog Neuro-Psychopharmacology Biol Psychiatry 79:369–377. https://doi.org/10.1016/j.pnpbp.2017.07.012
Silva RO, Damasceno SRB, Brito TV, Dias JM, Fontenele AM, Braúna IS et al (2015) Polysaccharide fraction isolated from Passiflora edulis inhibits the inflammatory response and the oxidative stress in mice. J Pharm Pharmacol 67:1017–1027. https://doi.org/10.1111/jphp.12399
Sisignano M, Baron R, Scholich K, Geisslinger G (2014) Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol 10:694–707. https://doi.org/10.1038/nrneurol.2014.211
Staff NP, Cavaletti G, Islam B, Lustberg M, Psimaras D, Tamburin S (2019) Platinum-induced peripheral neurotoxicity: from pathogenesis to treatment. J Peripher Nerv Syst 24:S26–S39. https://doi.org/10.1111/jns.12335
Starobova H, Vetter I (2017) Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci 10:174. https://doi.org/10.3389/fnmol.2017.00174
Vendel E, De Lange EC (2014) Functions of the CB1 and CB2 receptors in neuroprotection at the level of the blood-brain barrier. NeuroMolecular Med 16:620–642. https://doi.org/10.1007/s12017-014-8314-x
Viatchenko-Karpinski V, Ling J, Gu JG (2018) Down-regulation of Kv4.3 channels and a-type K+ currents in V2 trigeminal ganglion neurons of rats following oxaliplatin treatment Mol. Pain 14:1744806917750995. https://doi.org/10.1177/1744806917750995
Vučkovic S, Srebro D, Vujovic KS, Vučetic Č, Prostran M (2018) Cannabinoids and pain: new insights from old molecules. Front Pharmacol 9:1259. https://doi.org/10.3389/fphar.2018.01259
Waissengrin B, Mirelman D, Pelles S, Bukstein F, Blumenthal DT, Wolf I et al (2021) Effect of cannabis on oxaliplatin-induced peripheral neuropathy among oncology patients: a retrospective analysis. Ther Adv Med Oncol 13:1758835921990203. https://doi.org/10.1177/1758835921990203
Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA (2014) Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT 1A receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol 171:636–645. https://doi.org/10.1111/bph.12439
Ward SJ, Ramirez MD, Neelakantan H, Walker EA (2011) Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female c57bl6 mice. Anesth Analg 113:947–950. https://doi.org/10.1213/ANE.0b013e3182283486
Wilhelmsen K, Khakpour S, Tran A, Sheehan K, Schumacher M, Xu F et al (2014) The endocannabinoid/endovanilloid N-arachidonoyl dopamine (NADA) and synthetic cannabinoid WIN55,212–2 abate the inflammatory activation of human endothelial cells. J Biol Chem 289:13079–13100. https://doi.org/10.1074/jbc.M113.536953
Wu J, Hocevar M, Bie B, Foss JF, Naguib M (2019) Cannabinoid type 2 receptor system modulates paclitaxel-induced microglial dysregulation and central sensitization in rats. J Pain 20:501–514. https://doi.org/10.1016/j.jpain.2018.10.007
Xu DH, Cullen BD, Tang M, Fang Y (2020) The effectiveness of topical cannabidiol oil in symptomatic relief of peripheral neuropathy of the lower extremities. Curr Pharm Biotechnol 21:390–402. https://doi.org/10.2174/1389201020666191202111534
Acknowledgements
The authors would like to thank the Multi-User Facility of Drug Research and Development Center of Federal University of Ceará and the Central Analítica-UFC/CT-INFRA/MCTI-SISANO/Pró-Equipamentos CAPES for the technical support.
Funding
This study was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP) (Process PR2-0101–00054.01.00/15). This work was also partially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and by the Instituto Nacional de Ciência e Tecnologia Translacional em Medicina (INCT-TM; CNPq/FAPESP; 2008/09009–2); JAC received a grant from the University Global Partnership Network (UGPN) – Global Priorities in Cannabinoid Research Excellence Program. JEH, AWZ, JAC, RCPLJ, and MLV are recipients of CNPq research fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All procedures conducted in studies involving animals were in accordance with the ethical standards of the institution where the studies were performed. The study was approved by the Ethics Committee on the Use of Animals of the Federal University of Ceará (number: 41/2016).
Conflict of Interest
JAC is a member of the International Advisory Board of the Australian Centre for Cannabinoid Clinical and Research Excellence (ACRE) – National Health and Medical Research Council (NHMRC). JAC and JEH have received travel support to attend scientific meetings and personal consultation fees from BSPG-Pharm. JAC, JEH, and AWZ are coinventors of the patent “Fluorinated CBD compounds, compositions and uses thereof. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023,” Def. US number Reg. 62193296; July 29, 2015; INPI on August 19, 2015 (BR1120150164927; Mechoulam R, Zuardi AW, Kapczinski F, Hallak JEC, Guimarães FS, Crippa JAS, Breuer A). Universidade de São Paulo (USP) has licensed this patent to Phytecs Pharm (USP Resolution No. 15.1.130002.1.1) and has an agreement with Prati-Donaduzzi to “develop a pharmaceutical product containing synthetic CBD and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson’s disease, and anxiety disorders.” JAC, JEH, and AWZ are coinventors of the patent “Cannabinoid-containing oral pharmaceutical composition, method for preparing and using same,” INPI on September 16, 2016 (BR 112018005423–2). The other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12640_2021_442_MOESM1_ESM.tif
Supplementary file1 (TIF 16550 KB). Suppl. Fig. 1. Evaluation of cannabimimetic effects of cannabidiol. Catalepsy, hot plate, and rotarod tests were performed until the 56th experimental day to investigate the cannabimimetic effects of cannabidiol. Results are presented as mean ± SEM. n = 6. *P < 0.05 versus C + V (Two-way ANOVA, Tukey’s post-test). C: Control; CBD: Cannabidiol; OXL: Oxaliplatin; V: Vehicle.
12640_2021_442_MOESM2_ESM.tif
Supplementary file2 (TIF 16560 KB). Suppl. Fig. 2. The pharmacological modulation of cannabinoid receptors does not affect animals’ performance on the rota-rod test. Effect of WIN (a), AM251 (b), and AM630 (c) on the rota-rod test. Results are presented as mean ± SEM. n = 6. *P < 0.05 versus C + V (Two-way ANOVA, Tukey’s post-test). AM251: Cannabinoid CB1 receptor antagonist; AM630: Cannabinoid CB2 receptor antagonist; C: Control; OXL: Oxaliplatin; V: Vehicle; WIN: WIN 55,212-2.
12640_2021_442_MOESM3_ESM.tif
Supplementary file3 (TIF 70179 KB). Suppl. Fig. 3. Effect of cannabinoid receptor agonists or antagonists on CB2 expression in the dorsal root ganglia (DRG). Green: NeuN (neuronal marker); red: CB2; blue: DAPI (nuclear marker). Magnification: 200 ×. Scale bar: 50 μm. CB2 expression in DRG (a, b) on the 28th (28D) and 56th (56D) experimental days. The bar graphs present the mean ± SEM of the percentage of CB2 positive neurons compared to total neuronal cells. n = 6. *P < 0.05 versus control; §P < 0.05 versus OXL 28D; ^P < 0.05 versus OXL 56D (One-way ANOVA, Tukey’s post-test). AM251: Cannabinoid CB1 receptor antagonist; AM630: Cannabinoid CB2 receptor antagonist; CB1: Cannabinoid CB1 receptor; CB2: Cannabinoid CB2 receptor; CBD: Cannabidiol; OXL: oxaliplatin; WIN: WIN 55,212-2.
Rights and permissions
About this article
Cite this article
Pereira, A.F., Lisboa, M.R.P., de Freitas Alves, B.W. et al. Endocannabinoid System Attenuates Oxaliplatin-Induced Peripheral Sensory Neuropathy Through the Activation of CB1 Receptors. Neurotox Res 39, 1782–1799 (2021). https://doi.org/10.1007/s12640-021-00442-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00442-x