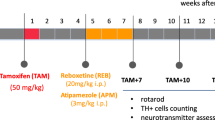
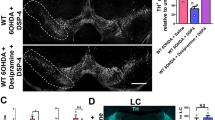
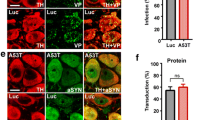
Abstract
It is now well accepted that there is a close relationship between noradrenergic and dopaminergic neurons in the brain, especially referring to the modulation of the locus coeruleus–norepinephrine (LC-NE) system on dopamine transmission. The disturbance of this modulation may contribute to neurodegeneration of dopaminergic neurons in Parkinson’s disease. In this article, we briefly review evidence related to such modulation. Firstly, we illustrated the noradrenergic innervation and functional implication for the LC-NE system and nigra–striatum dopaminergic system. Furthermore, we depicted neuroprotective effects of the LC-NE on dopaminergic neurons in vivo and in vitro. Moreover, we present data implicating the potential mechanisms underlying the modulation of the LC-NE system on dopaminergic neurons, in particular the effects of NE as a neurotrophic factor and through its ability to stimulate the expression of other neurotrophic factors, such as the brain-derived neurotrophic factor. Finally, we discussed other mechanisms intrinsic to NE’s effects. A better understanding of the noradrenergic modulation on dopaminergic neurons may be rewarding by significant advances in etiologic study and promising treatment of Parkinson’s disease.

Similar content being viewed by others
Abbreviations
- ARs:
-
Adrenergic receptors
- BDNF:
-
Brain-derived neurotrophic factor
- DA:
-
Dopamine
- CREB:
-
cAMP response element binding
- DBH:
-
Dopamine β-hydroxylase
- DSP4:
-
N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride
- LC:
-
Locus coeruleus
- NE:
-
Norepinephrine
- NET:
-
NE transporter
- PD:
-
Parkinson’s disease
- SNpc:
-
Substantia nigra pars compacta
- TrkB:
-
Tropomyosin receptor kinase B
- VTA:
-
Ventral tegmental area
References
Alonso-Vanegas MA, Fawcett JP, Causing CG, Miller FD, Sadikot AF (1999) Characterization of dopaminergic midbrain neurons in a DBH:BDNF transgenic mouse. J Comp Neurol 413:449–462
Aloyz R, Fawcett JP, Kaplan DR, Murphy RA, Miller FD (1999) Activity-dependent activation of TrkB neurotrophin receptors in the adult CNS. Learn Mem 6:216–231
Alvarez-Diduk R, Galano A (2015) Adrenaline and noradrenaline: protectors against oxidative stress or molecular targets? J Phys Chem B 119:3479–3491
Ancerewicz J, Migliavacca E, Carrupt PA et al (1998) Structure-property relationships of trimetazidine derivatives and model compounds as potential antioxidants. Free Radic Biol Med 25:113–120
Anden NE, Hfuxe K, Hamberger B, Hokfelt T (1966) A quantitative study on the nigro-neostriatal dopamine neuron system in the rat. Acta Physiol Scand 67:306–312
Anderson CF, Mosser DM (2002) A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol 72:101–106
Andorn AC, Pappolla MA (2001) Catecholamines inhibit lipid peroxidation in young, aged, and Alzheimer’s disease brain. Free Radic Biol Med 31:315–320
Antelman SM, Caggiula AR (1977) Norepinephrine-dopamine interactions and behavior. Science 195:646–653
Barde YA, Edgar D, Thoenen H (1982) Purification of a new neurotrophic factor from mammalian brain. EMBO J 1:549–553
Baydyuk M, Xu B (2014) BDNF signaling and survival of striatal neurons. Front Cell Neurosci 8:254
Berghuis P, Agerman K, Dobszay MB, Minichiello L, Harkany T, Ernfors P (2006) Brain-derived neurotrophic factor selectively regulates dendritogenesis of parvalbumin-containing interneurons in the main olfactory bulb through the PLCgamma pathway. J Neurobiol 66:1437–1451
Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42:33–84
Berridge CW, Stratford TL, Foote SL, Kelley AE (1997) Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse 27:230–241
Bing G, Zhang Y, Watanabe Y, McEwen BS, Stone EA (1994) Locus coeruleus lesions potentiate neurotoxic effects of MPTP in dopaminergic neurons of the substantia nigra. Brain Res 668:261–265
Bjorklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30:194–202
Blin J, Bonnet AM, Agid Y (1988) Does levodopa aggravate Parkinson’s disease? Neurology 38:1410–1416
Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME (1999) Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286:1358–1362
Brodal A (1981) Neurological anatomy in relation to clinical medicine. Oxford University Press, New York, pp 416–419
Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010) Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68:815–834
Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11:297–305
Buettner GR (1993) The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 300:535–543
Cabelli RJ, Hohn A, Shatz CJ (1995) Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science 267:1662–1666
Calabresi P, Giacomini P, Centonze D, Bernardi G (2000) Levodopa-induced dyskinesia: a pathological form of striatal synaptic plasticity? Ann Neurol 47:S60–S68 discussion S68-69
Castren E, Thoenen H, Lindholm D (1995) Brain-derived neurotrophic factor messenger RNA is expressed in the septum, hypothalamus and in adrenergic brain stem nuclei of adult rat brain and is increased by osmotic stimulation in the paraventricular nucleus. Neuroscience 64:71–80
Chen NH, Reith ME (1994) Autoregulation and monoamine interactions in the ventral tegmental area in the absence and presence of cocaine: a microdialysis study in freely moving rats. J Pharmacol Exp Ther 271:1597–1610
Chen MJ, Russo-Neustadt AA (2005) Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res Mol Brain Res 135:181–193
Chen MJ, Russo-Neustadt AA (2007) Nitric oxide signaling participates in norepinephrine-induced activity of neuronal intracellular survival pathways. Life Sci 81:1280–1290
Chen MJ, Nguyen TV, Pike CJ, Russo-Neustadt AA (2007) Norepinephrine induces BDNF and activates the PI-3K and MAPK cascades in embryonic hippocampal neurons. Cell Signal 19:114–128
Cohen Z, Molinatti G, Hamel E (1997) Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J Cereb Blood Flow Metab 17:894–904
Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S (1997) Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 17:2295–2313
Coradazzi M, Gulino R, Fieramosca F, Falzacappa LV, Riggi M, Leanza G (2016) Selective noradrenaline depletion impairs working memory and hippocampal neurogenesis. Neurobiol Aging 48:93–102
Cottingham C, Jones A, Wang Q (2012) Desipramine selectively potentiates norepinephrine-elicited ERK1/2 activation through the alpha2A adrenergic receptor. Biochem Biophys Res Commun 420:161–165
Counts SE, Mufson EJ (2010) Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J Neurochem 113:649–660
Culmsee C, Semkova I, Krieglstein J (1999) NGF mediates the neuroprotective effect of the beta2-adrenoceptor agonist clenbuterol in vitro and in vivo: evidence from an NGF-antisense study. Neurochem Int 35:47–57
da Penha Berzaghi M, Cooper J, Castren E, Zafra F, Sofroniew M, Thoenen H, Lindholm D (1993) Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3) mRNA levels in the developing rat hippocampus. J Neurosci 13:3818–3826
Datta SR, Brunet A, Greenberg ME (1999) Cellular survival: a play in three Akts. Genes Dev 13:2905–2927
Day JS, O’Neill E, Cawley C, Aretz NK, Kilroy D, Gibney SM, Harkin A, Connor TJ (2014) Noradrenaline acting on astrocytic beta(2)-adrenoceptors induces neurite outgrowth in primary cortical neurons. Neuropharmacology 77:234–248
Delaville C, Deurwaerdere PD, Benazzouz A (2011) Noradrenaline and Parkinson’s disease. Front Syst Neurosci 5:31
Dello Russo C, Boullerne AI, Gavrilyuk V, Feinstein DL (2004) Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1beta production. J Neuroinflammation 1:9
Dobolyi A, Vincze C, Pal G, Lovas G (2012) The neuroprotective functions of transforming growth factor beta proteins. Int J Mol Sci 13:8219–8258
Domesick VB (1988) Neuroanatomical organization of dopamine neurons in the ventral tegmental area. Ann N Y Acad Sci 537:10–26
Edeline JM, Manunta Y, Hennevin E (2011) Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear Res 274:75–84
Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, Manji HK, Chen G (2003) The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci 23:7311–7316
Elenkov IJ, Hasko G, Kovacs KJ, Vizi ES (1995) Modulation of lipopolysaccharide-induced tumor necrosis factor-alpha production by selective alpha- and beta-adrenergic drugs in mice. J Neuroimmunol 61:123–131
Encinas M, Iglesias M, Llecha N, Comella JX (1999) Extracellular-regulated kinases and phosphatidylinositol 3-kinase are involved in brain-derived neurotrophic factor-mediated survival and neuritogenesis of the neuroblastoma cell line SH-SY5Y. J Neurochem 73:1409–1421
Espay AJ, LeWitt PA, Kaufmann H (2014) Norepinephrine deficiency in Parkinson’s disease: the case for noradrenergic enhancement. Mov Disord 29:1710–1719
Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, McLean JH, Miller FD (1998) Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci 18:2808–2821
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5):2283–2301
Feinstein DL (1998) Suppression of astroglial nitric oxide synthase expression by norepinephrine results from decreased NOS-2 promoter activity. J Neurochem 70:1484–1496
Feinstein DL, Galea E, Reis DJ (1993) Norepinephrine suppresses inducible nitric oxide synthase activity in rat astroglial cultures. J Neurochem 60:1945–1948
Felten DL, Hallman H, Jonsson G (1982) Evidence for a neurotropic role of noradrenaline neurons in the postnatal development of rat cerebral cortex. J Neurocytol 11:119–135
Fornai F, Bassi L, Torracca MT, Scalori V, Corsini GU (1995) Norepinephrine loss exacerbates methamphetamine-induced striatal dopamine depletion in mice. Eur J Pharmacol 283:99–102
Fornai F, Torracca MT, Bassi L, D’Errigo DA, Scalori V, Corsini GU (1996) Norepinephrine loss selectively enhances chronic nigrostriatal dopamine depletion in mice and rats. Brain Res 735:349–353
Fornai F, Alessandri MG, Torracca MT, Bassi L, Scalori V, Corsini GU (1998) Noradrenergic modulation of methamphetamine-induced striatal dopamine depletion. Ann N Y Acad Sci 844:166–177
Fukuchi M, Fujii H, Takachi H, Ichinose H, Kuwana Y, Tabuchi A, Tsuda M (2010) Activation of tyrosine hydroxylase (TH) gene transcription induced by brain-derived neurotrophic factor (BDNF) and its selective inhibition through Ca(2+) signals evoked via the N-methyl-D-aspartate (NMDA) receptor. Brain Res 1366:18–26
Fulceri F, Biagioni F, Lenzi P, Falleni A, Gesi M, Ruggieri S, Fornai F (2006) Nigrostriatal damage with 6-OHDA: validation of routinely applied procedures. Ann N Y Acad Sci 1074:344–348
Fulceri F, Biagioni F, Ferrucci M, Lazzeri G, Bartalucci A, Galli V, Ruggieri S, Paparelli A, Fornai F (2007) Abnormal involuntary movements (AIMs) following pulsatile dopaminergic stimulation: severe deterioration and morphological correlates following the loss of locus coeruleus neurons. Brain Res 1135:219–229
Gesi M, Soldani P, Giorgi FS, Santinami A, Bonaccorsi I, Fornai F (2000) The role of the locus coeruleus in the development of Parkinson’s disease. Neurosci Biobehav Rev 24:655–668
Ghosh A, Carnahan J, Greenberg ME (1994) Requirement for BDNF in activity-dependent survival of cortical neurons. Science 263:1618–1623
Gleeson LC, Ryan KJ, Griffin EW, Connor TJ, Harkin A (2010) The beta2-adrenoceptor agonist clenbuterol elicits neuroprotective, anti-inflammatory and neurotrophic actions in the kainic acid model of excitotoxicity. Brain Behav Immun 24:1354–1361
Gorski JA, Zeiler SR, Tamowski S, Jones KR (2003) Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci 23:6856–6865
Gozdz A, Habas A, Jaworski J, Zielinska M, Albrecht J, Chlystun M, Jalili A, Hetman M (2003) Role of N-methyl-D-aspartate receptors in the neuroprotective activation of extracellular signal-regulated kinase 1/2 by cisplatin. J Biol Chem 278:43663–43671
Grailer JJ, Haggadone MD, Sarma JV, Zetoune FS, Ward PA (2014) Induction of M2 regulatory macrophages through the beta2-adrenergic receptor with protection during endotoxemia and acute lung injury. J Innate Immun 6:607–618
Grenhoff J, Svensson TH (1993) Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur J Pharmacol 233:79–84
Grenhoff J, Nisell M, Ferre S, Aston-Jones G, Svensson TH (1993) Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect 93:11–25
Guiard BP, El Mansari M, Merali Z, Blier P (2008) Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol 11:625–639
Haring JH, Davis JN (1985) Differential distribution of locus coeruleus projections to the hippocampal formation: anatomical and biochemical evidence. Brain Res 325:366–369
Hasko G, Shanley TP, Egnaczyk G, Nemeth ZH, Salzman AL, Vizi ES, Szabo C (1998) Exogenous and endogenous catecholamines inhibit the production of macrophage inflammatory protein (MIP) 1 alpha via a beta adrenoceptor mediated mechanism. Br J Pharmacol 125:1297–1303
Heerssen HM, Segal RA (2002) Location, location, location: a spatial view of neurotrophin signal transduction. Trends Neurosci 25:160–165
Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O’Banion MK, Weinberg G, Klockgether T, Feinstein DL (2002) Noradrenergic depletion potentiates beta-amyloid-induced cortical inflammation: implications for Alzheimer’s disease. J Neurosci 22:2434–2442
Henry B, Fox SH, Peggs D, Crossman AR, Brotchie JM (1999) The alpha2-adrenergic receptor antagonist idazoxan reduces dyskinesia and enhances anti-parkinsonian actions of L-dopa in the MPTP-lesioned primate model of Parkinson’s disease. Mov Disord 14:744–753
Hetman M, Kanning K, Cavanaugh JE, Xia Z (1999) Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem 274:22569–22580
Hillman KL, Lei S, Doze VA, Porter JE (2009) Alpha-1A adrenergic receptor activation increases inhibitory tone in CA1 hippocampus. Epilepsy Res 84:97–109
Hirata H, Ladenheim B, Carlson E, Epstein C, Cadet JL (1996) Autoradiographic evidence for methamphetamine-induced striatal dopaminergic loss in mouse brain: attenuation in CuZn-superoxide dismutase transgenic mice. Brain Res 714:95–103
Hirsch E, Graybiel AM, Agid YA (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 334:345–348
Hotchkiss AJ, Gibb JW (1980) Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther 214:257–262
Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R (2007) Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 131:160–173
Huang YY, Peng CH, Yang YP et al (2007) Desipramine activated Bcl-2 expression and inhibited lipopolysaccharide-induced apoptosis in hippocampus-derived adult neural stem cells. J Pharmacol Sci 104:61–72
Huang YZ, Pan E, Xiong ZQ, McNamara JO (2008) Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron 57:546–558
Inoue S, Susukida M, Ikeda K, Murase K, Hayashi K (1997) Dopaminergic transmitter up-regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) synthesis in mouse astrocytes in culture. Biochem Biophys Res Commun 238:468–472
Isaias IU, Marotta G, Pezzoli G, Sabri O, Schwarz J, Crenna P, Classen J, Cavallari P (2011) Enhanced catecholamine transporter binding in the locus coeruleus of patients with early Parkinson disease. BMC Neurol 11:88
Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA (2003) Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol Biochem Behav 75:81–88
Jhang KA, Lee EO, Kim HS, Chong YH (2014) Norepinephrine provides short-term neuroprotection against Abeta1-42 by reducing oxidative stress independent of Nrf2 activation. Neurobiol Aging 35:2465–2473
Jhaveri DJ, Mackay EW, Hamlin AS, Marathe SV, Nandam LS, Vaidya VA, Bartlett PF (2010) Norepinephrine directly activates adult hippocampal precursors via beta3-adrenergic receptors. J Neurosci 30:2795–2806
Jones LS, Gauger LL, Davis JN (1985) Anatomy of brain alpha 1-adrenergic receptors: in vitro autoradiography with [125I]-heat. J Comp Neurol 231:190–208
Jones KR, Farinas I, Backus C, Reichardt LF (1994) Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 76:989–999
Juric DM, Miklic S, Carman-Krzan M (2006) Monoaminergic neuronal activity up-regulates BDNF synthesis in cultured neonatal rat astrocytes. Brain Res 1108:54–62
Juric DM, Loncar D, Carman-Krzan M (2008) Noradrenergic stimulation of BDNF synthesis in astrocytes: mediation via alpha1- and beta1/beta2-adrenergic receptors. Neurochem Int 52:297–306
Kajitani N, Hisaoka-Nakashima K, Morioka N, Okada-Tsuchioka M, Kaneko M, Kasai M, Shibasaki C, Nakata Y, Takebayashi M (2012) Antidepressant acts on astrocytes leading to an increase in the expression of neurotrophic/growth factors: differential regulation of FGF-2 by noradrenaline. PLoS One 7:e51197
Kalashnikova EV, Dymshits G, Kolpakov FA (2006) Signal transduction pathways involved in transcriptional regulation of tyrosine hydroxylase. In: Fight International Conference on Bioinformatics of Genome Regulation and Structure-BGRS’2006, pp. 128–131. Novosibirsk
Kalinin S, Feinstein DL, Xu HL, Huesa G, Pelligrino DA, Galea E (2006) Degeneration of noradrenergic fibres from the locus coeruleus causes tight-junction disorganisation in the rat brain. Eur J Neurosci 24:3393–3400
Kang YC, Kim PK, Choi BM, Chung HT, Ha KS, Kwon YG, Kim YM (2004) Regulation of programmed cell death in neuronal cells by nitric oxide. In Vivo 18:367–376
Katsuki H, Izumi Y, Zorumski CF (1997) Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol 77:3013–3020
Kilbourn MR, Sherman P, Abbott LC (1998) Reduced MPTP neurotoxicity in striatum of the mutant mouse tottering. Synapse 30:205–210
Kim KS, Lee MK, Carroll J, Joh TH (1993) Both the basal and inducible transcription of the tyrosine hydroxylase gene are dependent upon a cAMP response element. J Biol Chem 268:15689–15695
Kreider ML, Seidler FJ, Cousins MM, Tate CA, Slotkin TA (2004) Transiently overexpressed alpha2-adrenoceptors and their control of DNA synthesis in the developing brain. Brain Res Dev Brain Res 152:233–239
Kulkarni VA, Jha S, Vaidya VA (2002) Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur J Neurosci 16:2008–2012
Laifenfeld D, Klein E, Ben-Shachar D (2002) Norepinephrine alters the expression of genes involved in neuronal sprouting and differentiation: relevance for major depression and antidepressant mechanisms. J Neurochem 83:1054–1064
Lategan AJ, Marien MR, Colpaert FC (1990) Effects of locus coeruleus lesions on the release of endogenous dopamine in the rat nucleus accumbens and caudate nucleus as determined by intracerebral microdialysis. Brain Res 523:134–138
Lategan AJ, Marien MR, Colpaert FC (1992) Suppression of nigrostriatal and mesolimbic dopamine release in vivo following noradrenaline depletion by DSP-4: a microdialysis study. Life Sci 50:995–999
Lauder JM (1993) Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci 16:233–240
Lee FS, Chao MV (2001) Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci U S A 98:3555–3560
Lee A, Wissekerke AE, Rosin DL, Lynch KR (1998) Localization of alpha2C-adrenergic receptor immunoreactivity in catecholaminergic neurons in the rat central nervous system. Neuroscience 84:1085–1096
Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV (2002) Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem 277:9096–9102
Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde YA (1989) Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341:149–152
Lidow MS, Rakic P (1994) Unique profiles of the alpha 1-, alpha 2-, and beta-adrenergic receptors in the developing cortical plate and transient embryonic zones of the rhesus monkey. J Neurosci 14:4064–4078
Lim J, Yang C, Hong SJ, Kim KS (2000) Regulation of tyrosine hydroxylase gene transcription by the cAMP-signaling pathway: involvement of multiple transcription factors. Mol Cell Biochem 212:51–60
Lindholm D, Castren E, Berzaghi M, Blochl A, Thoenen H (1994) Activity-dependent and hormonal regulation of neurotrophin mRNA levels in the brain—implications for neuronal plasticity. J Neurobiol 25:1362–1372
Linner L, Endersz H, Ohman D, Bengtsson F, Schalling M, Svensson TH (2001) Reboxetine modulates the firing pattern of dopamine cells in the ventral tegmental area and selectively increases dopamine availability in the prefrontal cortex. J Pharmacol Exp Ther 297:540–546
Liprando LA, Miner LH, Blakely RD, Lewis DA, Sesack SR (2004) Ultrastructural interactions between terminals expressing the norepinephrine transporter and dopamine neurons in the rat and monkey ventral tegmental area. Synapse 52:233–244
Liu J, Mori A (1993) Monoamine metabolism provides an antioxidant defense in the brain against oxidant- and free radical-induced damage. Arch Biochem Biophys 302:118–127
Liu Y, Formisano L, Savtchouk I, Takayasu Y, Szabo G, Zukin RS, Liu SJ (2010) A single fear-inducing stimulus induces a transcription-dependent switch in synaptic AMPAR phenotype. Nat Neurosci 13:223–231
Liu X, Ye K, Weinshenker D (2015) Norepinephrine protects against amyloid-beta toxicity via TrkB. J Alzheimers Dis 44:251–260
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15:120–132
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110
Marien M, Briley M, Colpaert F (1993) Noradrenaline depletion exacerbates MPTP-induced striatal dopamine loss in mice. Eur J Pharmacol 236:487–489
Marien M, Lategan A, Colpaert F (1994) Noradrenergic control of striatal dopamine. In: Briley M, Marien M (eds) Noradrenergic mechanisms in Parkinson’s disease. CRC Press, Boca Raton, pp 127–138
Martel J, Chopin P, Colpaert F, Marien M (1998) Neuroprotective effects of the alpha2-adrenoceptor antagonists, (+)-efaroxan and (+/−)-idazoxan, against quinolinic acid-induced lesions of the rat striatum. Exp Neurol 154:595–601
Matsumoto M, Hikosaka O (2009) Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459:837–841
Mavridis M, Degryse AD, Lategan AJ, Marien MR, Colpaert FC (1991) Effects of locus coeruleus lesions on parkinsonian signs, striatal dopamine and substantia nigra cell loss after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in monkeys: a possible role for the locus coeruleus in the progression of Parkinson’s disease. Neuroscience 41:507–523
McAllister AK, Lo DC, Katz LC (1995) Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15:791–803
McAllister AK, Katz LC, Lo DC (1996) Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 17:1057–1064
McAllister AK, Katz LC, Lo DC (1999) Neurotrophins and synaptic plasticity. Annu Rev Neurosci 22:295–318
Mejias-Aponte CA (2016) Specificity and impact of adrenergic projections to the midbrain dopamine system. Brain Res 1641:258–273
Mejias-Aponte CA, Drouin C, Aston-Jones G (2009) Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci 29:3613–3626
Miklic S, Juric DM, Carman-Krzan M (2004) Differences in the regulation of BDNF and NGF synthesis in cultured neonatal rat astrocytes. Int J Dev Neurosci 22:119–130
Mittal S, Bjornevik K, Im DS et al (2017) beta2-Adrenoreceptor is a regulator of the alpha-synuclein gene driving risk of Parkinson’s disease. Science 357:891–898
Moore RY, Bloom FE (1979) Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci 2:113–168
Morrison JH, Molliver ME, Grzanna R (1979) Noradrenergic innervation of cerebral cortex: widespread effects of local cortical lesions. Science 205:313–316
Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE (2012) Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb Perspect Med 2:a009381
Musazzi L, Rimland JM, Ieraci A, Racagni G, Domenici E, Popoli M (2014) Pharmacological characterization of BDNF promoters I, II and IV reveals that serotonin and norepinephrine input is sufficient for transcription activation. Int J Neuropsychopharmacol 17:779–791
Nagamoto-Combs K, Piech KM, Best JA, Sun B, Tank AW (1997) Tyrosine hydroxylase gene promoter activity is regulated by both cyclic AMP-responsive element and AP1 sites following calcium influx. Evidence for cyclic amp-responsive element binding protein-independent regulation. J Biol Chem 272:6051–6058
Nawa H, Pelleymounter MA, Carnahan J (1994) Intraventricular administration of BDNF increases neuropeptide expression in newborn rat brain. J Neurosci 14:3751–3765
Noh JS, Kim EY, Kang JS, Kim HR, Oh YJ, Gwag BJ (1999) Neurotoxic and neuroprotective actions of catecholamines in cortical neurons. Exp Neurol 159:217–224
Numan S, Lane-Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB, Nestler EJ (1998) Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci 18:10700–10708
O’Sullivan JB, Ryan KM, Curtin NM, Harkin A, Connor TJ (2009) Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int J Neuropsychopharmacol 12:687–699
O’Sullivan JB, Ryan KM, Harkin A, Connor TJ (2010) Noradrenaline reuptake inhibitors inhibit expression of chemokines IP-10 and RANTES and cell adhesion molecules VCAM-1 and ICAM-1 in the CNS following a systemic inflammatory challenge. J Neuroimmunol 220:34–42
Oades RD, Halliday GM (1987) Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res 434:117–165
Orefice LL, Waterhouse EG, Partridge JG, Lalchandani RR, Vicini S, Xu B (2013) Distinct roles for somatically and dendritically synthesized brain-derived neurotrophic factor in morphogenesis of dendritic spines. J Neurosci 33:11618–11632
Patel NJ, Chen MJ, Russo-Neustadt AA (2010) Norepinephrine and nitric oxide promote cell survival signaling in hippocampal neurons. Eur J Pharmacol 633:1–9
Picconi B, Pisani A, Barone I, Bonsi P, Centonze D, Bernardi G, Calabresi P (2005) Pathological synaptic plasticity in the striatum: implications for Parkinson’s disease. Neurotoxicology 26:779–783
Popovik E, Haynes LW (2000) Survival and mitogenesis of neuroepithelial cells are influenced by noradrenergic but not cholinergic innervation in cultured embryonic rat neopallium. Brain Res 853:227–235
Rajagopal R, Chen ZY, Lee FS, Chao MV (2004) Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci 24:6650–6658
Rascol O, Arnulf I, Peyro-Saint Paul H et al (2001) Idazoxan, an alpha-2 antagonist, and L-DOPA-induced dyskinesias in patients with Parkinson’s disease. Mov Disord 16:708–713
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc London Ser B Biol Sci 361:1545–1564
Robbins TW (2000) From arousal to cognition: the integrative position of the prefrontal cortex. Prog Brain Res 126:469–483
Robbins T, Everitt B (1995) Central norepinephrine neurons and behavior. In: Bloom F, Kupfer D (eds) Neuropsychopharmacology: the fourth generation of progress. Raven Press, New York
Rommelfanger KS, Weinshenker D, Miller GW (2004) Reduced MPTP toxicity in noradrenaline transporter knockout mice. J Neurochem 91:1116–1124
Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D (2007) Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci U S A 104:13804–13809
Ross RA, Reis DJ (1974) Effects of lesions of locus coeruleus on regional distribution of dopamine-beta-hydroxylase activity in rat brain. Brain Res 73:161–166
Ruiz CR, Shi J, Meffert MK (2014) Transcript specificity in BDNF-regulated protein synthesis. Neuropharmacology 76(Pt C):657–663
Samuels ER, Szabadi E (2008) Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol 6:235–253
Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10:211–223
Satoh T, Nakai S, Sato T, Kimura M (2003) Correlated coding of motivation and outcome of decision by dopamine neurons. J Neurosci 23:9913–9923
Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD (2000) Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol 420:211–232
Schwartz JP, Nishiyama N (1994) Neurotrophic factor gene expression in astrocytes during development and following injury. Brain Res Bull 35:403–407
Schwartz JP, Nishiyama N, Wilson D, Taniwaki T (1994) Receptor-mediated regulation of neuropeptide gene expression in astrocytes. Glia 11:185–190
Sciarretta C, Fritzsch B, Beisel K, Rocha-Sanchez SM, Buniello A, Horn JM, Minichiello L (2010) PLCgamma-activated signalling is essential for TrkB mediated sensory neuron structural plasticity. BMC Dev Biol 10:103
Segal M, Bloom FE (1976) The action of norepinephrine in the rat hippocampus. III. Hippocampal cellular responses to locus coeruleus stimulation in the awake rat. Brain Res 107:499–511
Segal RA (2003) Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci 26:299–330
Shaywitz AJ, Greenberg ME (1999) CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68:821–861
Shepard KN, Liles LC, Weinshenker D, Liu RC (2015) Norepinephrine is necessary for experience-dependent plasticity in the developing mouse auditory cortex. J Neurosci 35:2432–2437
Siciliano R, Fornai F, Bonaccorsi I, Domenici L, Bagnoli P (1999) Cholinergic and noradrenergic afferents influence the functional properties of the postnatal visual cortex in rats. Vis Neurosci 16:1015–1028
Simon H, Le Moal M, Stinus L, Calas A (1979) Anatomical relationships between the ventral mesencephalic tegmentum—a 10 region and the locus coeruleus as demonstrated by anterograde and retrograde tracing techniques. J Neural Transm 44:77–86
Smith MA, Makino S, Altemus M, Michelson D, Hong SK, Kvetnansky R, Post RM (1995) Stress and antidepressants differentially regulate neurotrophin 3 mRNA expression in the locus coeruleus. Proc Natl Acad Sci U S A 92:8788–8792
Spengler RN, Chensue SW, Giacherio DA, Blenk N, Kunkel SL (1994) Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J Immunol 152:3024–3031
Srinivasan J, Schmidt WJ (2003) Potentiation of parkinsonian symptoms by depletion of locus coeruleus noradrenaline in 6-hydroxydopamine-induced partial degeneration of substantia nigra in rats. Eur J Neurosci 17:2586–2592
Sterpenich V, D’Argembeau A, Desseilles M et al (2006) The locus ceruleus is involved in the successful retrieval of emotional memories in humans. J Neurosci 26:7416–7423
Stroemer RP, Kent TA, Hulsebosch CE (1998) Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke 29:2381–2393 discussion 2393-2385
Sullivan RM, Wilson DA, Leon M (1989) Norepinephrine and learning-induced plasticity in infant rat olfactory system. J Neurosci 9:3998–4006
Sullivan RM, McGaugh JL, Leon M (1991) Norepinephrine-induced plasticity and one-trial olfactory learning in neonatal rats. Brain Res Dev Brain Res 60:219–228
Suzuki T, Kurahashi H, Ichinose H (2004) Ras/MEK pathway is required for NGF-induced expression of tyrosine hydroxylase gene. Biochem Biophys Res Commun 315:389–396
Swanson LW (1982) The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 9:321–353
Swanson LW, Hartman BK (1975) The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol 163:467–505
Szabo C, Hasko G, Zingarelli B, Nemeth ZH, Salzman AL, Kvetan V, Pastores SM, Vizi ES (1997) Isoproterenol regulates tumour necrosis factor, interleukin-10, interleukin-6 and nitric oxide production and protects against the development of vascular hyporeactivity in endotoxaemia. Immunology 90:95–100
Takahashi M, Niki E (1998) The effect of oxidative stress on cells by oxygen radicals and its inhibition by antioxidants. In: Montagnier L, Olivier R, Pasquier F (eds) Oxidative stress in cancer, AIDA and neurodegenerative diseases. Marcel Decker, New York, pp 9–14
Thastrup O, Knudsen JB, Lemmich J, Winther K (1985) Inhibition of human platelet aggregation by dihydropyrano- and dihydrofuranocoumarins, a new class of cAMP-phosphodiesterase inhibitors. Biochem Pharmacol 34:2137–2140
Thoenen H (1995) Neurotrophins and neuronal plasticity. Science 270:593–598
Timmer M, Cesnulevicius K, Winkler C, Kolb J, Lipokatic-Takacs E, Jungnickel J, Grothe C (2007) Fibroblast growth factor (FGF)-2 and FGF receptor 3 are required for the development of the substantia nigra, and FGF-2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxydopamine lesion. J Neurosci 27:459–471
Tinti C, Conti B, Cubells JF, Kim KS, Baker H, Joh TH (1996) Inducible cAMP early repressor can modulate tyrosine hydroxylase gene expression after stimulation of cAMP synthesis. J Biol Chem 271:25375–25381
Tong J, Hornykiewicz O, Kish SJ (2006) Inverse relationship between brain noradrenaline level and dopamine loss in Parkinson disease: a possible neuroprotective role for noradrenaline. Arch Neurol 63:1724–1728
Traver S, Salthun-Lassalle B, Marien M, Hirsch EC, Colpaert F, Michel PP (2005) The neurotransmitter noradrenaline rescues septal cholinergic neurons in culture from degeneration caused by low-level oxidative stress. Mol Pharmacol 67:1882–1891
Troadec JD, Marien M, Darios F, Hartmann A, Ruberg M, Colpaert F, Michel PP (2001) Noradrenaline provides long-term protection to dopaminergic neurons by reducing oxidative stress. J Neurochem 79:200–210
Troadec JD, Marien M, Mourlevat S, Debeir T, Ruberg M, Colpaert F, Michel PP (2002) Activation of the mitogen-activated protein kinase (ERK(1/2)) signaling pathway by cyclic AMP potentiates the neuroprotective effect of the neurotransmitter noradrenaline on dopaminergic neurons. Mol Pharmacol 62:1043–1052
Vauquelin G, Geynet P, Hanoune J, Strosberg AD (1979) Affinity chromatography of the beta-adrenergic receptor from Turkey erythrocytes. Eur J Biochem 98:543–556
Vijayashankar N, Brody H (1979) A quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. J Neuropathol Exp Neurol 38:490–497
Vitalis T, Cases O, Parnavelas JG (2005) Development of the dopaminergic neurons in the rodent brainstem. Exp Neurol 191(Suppl 1):S104–S112
Vizi ES, Orso E, Osipenko ON, Hasko G, Elenkov IJ (1995) Neurochemical, electrophysiological and immunocytochemical evidence for a noradrenergic link between the sympathetic nervous system and thymocytes. Neuroscience 68:1263–1276
Wang Y, Zhang QJ, Liu J, Ali U, Gui ZH, Hui YP, Chen L, Wu ZH, Li Q (2010) Noradrenergic lesion of the locus coeruleus increases apomorphine-induced circling behavior and the firing activity of substantia nigra pars reticulata neurons in a rat model of Parkinson’s disease. Brain Res 1310:189–199
Weinshenker D, Ferrucci M, Busceti CL et al (2008) Genetic or pharmacological blockade of noradrenaline synthesis enhances the neurochemical, behavioral, and neurotoxic effects of methamphetamine. J Neurochem 105:471–483
Widmer HR, Kaplan DR, Rabin SJ, Beck KD, Hefti F, Knusel B (1993) Rapid phosphorylation of phospholipase C gamma 1 by brain-derived neurotrophic factor and neurotrophin-3 in cultures of embryonic rat cortical neurons. J Neurochem 60:2111–2123
Xing B, Xin T, Zhao L, Hunter RL, Chen Y, Bing G (2010) Glial cell line-derived neurotrophic factor protects midbrain dopaminergic neurons against lipopolysaccharide neurotoxicity. J Neuroimmunol 225:43–51
Yamada M, Ohnishi H, Sano S, Araki T, Nakatani A, Ikeuchi T, Hatanaka H (1999) Brain-derived neurotrophic factor stimulates interactions of Shp2 with phosphatidylinositol 3-kinase and Grb2 in cultured cerebral cortical neurons. J Neurochem 73:41–49
Yanpallewar SU, Fernandes K, Marathe SV et al (2010) Alpha2-adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment. J Neurosci 30:1096–1109
Yuan J, Yankner BA (2000) Apoptosis in the nervous system. Nature 407:802–809
Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H (1992) Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci 12:4793–4799
Zarow C, Lyness SA, Mortimer JA, Chui HC (2003) Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 60:337–341
Zuccato C, Cattaneo E (2009) Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 5:311–322
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhu, MY. Noradrenergic Modulation on Dopaminergic Neurons. Neurotox Res 34, 848–859 (2018). https://doi.org/10.1007/s12640-018-9889-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9889-z