Abstract
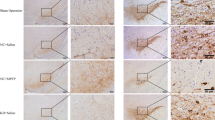
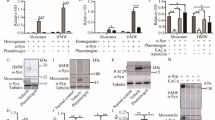
Parkinson’s disease (PD) is characterized by a triade of motor symptoms due to the degeneration of nigrostriatal pathway. In addition to these motor impairments, cognitive disturbances have been reported to occur in PD patients in the early stage of the disease. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a neurotoxin widely used to produce experimental models of PD. In a previous work, we showed that MPTP altered the expression of proteins involved in mTOR antiapoptotic and PKR apoptotic pathways of translational control (TC) in neuroblastoma cells. In the present study, the results indicated that a subchronic MPTP intoxication in mice decreased the dopaminergic neuron number, produced an activation of PKR way and an inhibition of mTOR way of TC especially in striatum and frontal cortex associated with a great activation of PKR in hippocampus. Moreover, in parallel to biochemical analysis, the mnesic disturbances induced by MPTP were characterized in C57Bl/6 mice, by testing their performance in three versions of the Morris Water Maze task. Behavioral results showed that the MPTP lesion altered mice learning of a spatial working memory, of a cued version and of a spatial reference memory task in the water maze. Furthermore, we previously demonstrated that the neuropeptide pituitary adenylate cyclase activating polypeptide (PACAP) could counteract the MPTP toxicity on TC factors in neuroblastoma cells. Thus, the second objective of our study was to assess the PACAP effect on MPTP-induced TC impairment and cognitive deficit in mice. The pretreatment with PACAP27 by intravenous injections partially protected TH-positive neuron loss induced by MPTP, prevented the MPTP-induced protein synthesis control dysregulation and mnesic impairment of mice. Therefore, our results could indicate that PACAP may be a promising therapeutic agent in Parkinson’s disease.




Similar content being viewed by others
Abbreviations
- 4E-BP1:
-
4E-Binding protein 1
- eIF2α:
-
Eukaryotic initiation factor 2α
- eIF4E:
-
Eukaryotic initiation factor 4E
- FBS:
-
Fetal bovine serum
- mTOR:
-
Mammalian target of rapamycin
- PACAP:
-
Pituitary adenylate cyclase activating polypeptide
- PFC:
-
Prefrontal cortex
- PKR:
-
Double-stranded RNA-protein dependent kinase
- p70S6K:
-
Ribosomal p70S6 kinase
- MPP+ :
-
1-Methyl-4-phenylpyridinium ion
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD:
-
Parkinson’s disease
- SN:
-
Substantia nigra
- TH:
-
Tyrosine hydroxylase
References
Arimura A, Somogyvari-Vigh A, Weill C, Fiore RC, Tatsuno I, Bay V, Brenneman DE (1994) PACAP functions as a neurotrophic factor. Ann NY Acad Sci 739:228–243
Balachandran S, Kim CN, Yeh WC, Mak TW, Bhalla K, Barber GN (1998) Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J 17:6888–6902
Bando Y, Onuki R, Katayama T, Manabe T, Kudo T, Taira K, Tohyama M (2005) Double-strand RNA dependent protein kinase (PKR) is involved in the extrastriatal degeneration in Parkinson’s disease and Huntington’s disease. Neurochem Int 46:11–18
Banks WA, Uchida D, Arimura A, Somogyvari-Vigh A, Shioda S (1996) Transport of pituitary adenylate cyclase-activating polypeptide across the blood-brain barrier and the prevention of ischemia-induced death of hippocampal neurons. Ann NY Acad Sci 805:270–277; discussion 277–279
Bobrovskaya L, Gelain DP, Gilligan C, Dickson PW, Dunkley PR (2007) PACAP stimulates the sustained phosphorylation of tyrosine hydroxylase at serine 40. Cell Signal 19:1141–1149
Brozoski TJ, Brown RM, Rosvold HE, Goldman PS (1979) Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205:929–932
Bruck A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO (2004) Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry 75:1467–1469
Bubser M, Schmidt WJ (1990) 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav Brain Res 37:157–168
Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP (2003) Time-restricted role for dendritic activation of the mTOR-p70S6 K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci USA 100:14368–14373
Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O (2005) Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet 14:1709–1725
Clemens MJ (2001) Translational regulation in cell stress and apoptosis. Roles of the eIF4E binding proteins. J Cell Mol Med 5:221–239
Cooper JA, Sagar HJ, Doherty SM, Jordan N, Tidswell P, Sullivan EV (1992) Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson’s disease. A follow-up study of untreated patients. Brain 115(Pt 6):1701–1725
Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M, Imataka H, Cuello AC, Seidah N, Sossin W, Lacaille JC, Ron D, Nader K, Sonenberg N (2005) Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature 436:1166–1173
Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjevic K, Lacaille JC, Nader K, Sonenberg N (2007) eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129:195–206
Da Cunha C, Angelucci ME, Canteras NS, Wonnacott S, Takahashi RN (2002) The lesion of the rat substantia nigra pars compacta dopaminergic neurons as a model for Parkinson’s disease memory disabilities. Cell Mol Neurobiol 22:227–237
Da Cunha C, Gevaerd MS, Vital MA, Miyoshi E, Andreatini R, Silveira R, Takahashi RN, Canteras NS (2001) Memory disruption in rats with nigral lesions induced by MPTP: a model for early Parkinson's disease amnesia. Behav Brain Res 124:9–18
Da Cunha C, Wietzikoski S, Wietzikoski EC, Miyoshi E, Ferro MM, Anselmo-Franci JA, Canteras NS (2003) Evidence for the substantia nigra pars compacta as an essential component of a memory system independent of the hippocampal memory system. Neurobiol Learn Mem 79:236–242
Deguil J, Jailloux D, Page G, Fauconneau B, Houeto JL, Philippe M, Muller JM, Pain S (2007) Neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) in MPP+ -induced alteration of translational control in Neuro-2a neuroblastoma cells. J Neurosci Res 85:2017–2025
Der SD, Yang YL, Weissmann C, Williams BR (1997) A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA 94:3279–3283
Dogrukol-Ak D, Tore F, Tuncel N (2004) Passage of VIP/PACAP/secretin family across the blood-brain barrier: therapeutic effects. Curr Pharm Des 10:1325–1340
Dubois B, Pillon B (1997) Cognitive deficits in Parkinson’s disease. J Neurol 244:2–8
Dufner A, Thomas G (1999) Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 253:100–109
Farkas O, Tamas A, Zsombok A, Reglodi D, Pal J, Buki A, Lengvari I, Povlishock JT, Doczi T (2004) Effects of pituitary adenylate cyclase activating polypeptide in a rat model of traumatic brain injury. Regul Pept 123:69–75
Ferro MM, Bellissimo MI, Anselmo-Franci JA, Angellucci ME, Canteras NS, Da Cunha C (2005) Comparison of bilaterally 6-OHDA- and MPTP-lesioned rats as models of the early phase of Parkinson’s disease: histological, neurochemical, motor and memory alterations. J Neurosci Methods 148:78–87
Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL (1995) Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging 16:149–160
Gerlach M, Riederer P (1996) Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm 103:987–1041
Gerlach M, Double KL, Youdim MB, Riederer P (2000) Strategies for the protection of dopaminergic neurons against neurotoxicity. Neurotox Res 2:99–114
Gevaerd MS, Miyoshi E, Silveira R, Canteras NS, Takahashi RN, Da Cunha C (2001) L-Dopa restores striatal dopamine level but fails to reverse MPTP-induced memory deficits in rats. Int J Neuropsychopharmacol 4:361–370
Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N (2001) Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev 15:2852–2864
Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC (2000) Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci USA 97:2875–2880
Kesner RP (2000) Behavioral analysis of the contribution of the hippocampus and parietal cortex to the processing of information: interactions and dissociations. Hippocampus 10:483–490
Kostrzewa RM, Segura-Aguilar J (2002) Neurotoxicological and neuroprotective elements in Parkinson’s disease. Neurotox Res 4:83–86
Lafay-Chebassier C, Paccalin M, Page G, Barc-Pain S, Perault-Pochat MC, Gil R, Pradier L, Hugon J (2005) mTOR/p70S6k signalling alteration by Abeta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer’s disease. J Neurochem 94:215–225
Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM (1992) L-dopa withdrawal in Parkinson’s disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology (Berl) 107:394–404
Lees AJ, Smith E (1983) Cognitive deficits in the early stages of Parkinson’s disease. Brain 106(Pt 2):257–270
Levin BE, Llabre MM, Weiner WJ (1989) Cognitive impairments associated with early Parkinson’s disease. Neurology 39:557–561
Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM (2003) Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci 23:6351–6356
Malagelada C, Ryu EJ, Biswas SC, Jackson-Lewis V, Greene LA (2006) RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson’s disease by a mechanism involving mammalian target of rapamycin inactivation. J Neurosci 26:9996–10005
Masuo Y, Matsumoto Y, Tokito F, Tsuda M, Fujino M (1993) Effects of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) on the spontaneous release of acetylcholine from the rat hippocampus by brain microdialysis. Brain Res 611:207–215
Miyoshi E, Wietzikoski S, Camplessei M, Silveira R, Takahashi RN, Da Cunha C (2002) Impaired learning in a spatial working memory version and in a cued version of the water maze in rats with MPTP-induced mesencephalic dopaminergic lesions. Brain Res Bull 58:41–47
Mochizuki H, Goto K, Mori H, Mizuno Y (1996) Histochemical detection of apoptosis in Parkinson’s disease. J Neurol Sci 137:120–123
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Mura A, Feldon J (2003) Spatial learning in rats is impaired after degeneration of the nigrostriatal dopaminergic system. Mov Disord 18:860–871
Onoue S, Endo K, Ohshima K, Yajima T, Kashimoto K (2002) The neuropeptide PACAP attenuates beta-amyloid (1-42)-induced toxicity in PC12 cells. Peptides 23:1471–1478
Owen AM, Iddon JL, Hodges JR, Summers BA, Robbins TW (1997) Spatial and non-spatial working memory at different stages of Parkinson’s disease. Neuropsychologia 35:519–532
Paccalin M, Pain-Barc S, Pluchon C, Paul C, Bazin H, Gil R, Hugon J (2005) The relation between p70S6k expression in lymphocytes and the decline of cognitive test scores in patients with Alzheimer disease. Arch Intern Med 165:2428–2429
Packard MG, McGaugh JL (1992) Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci 106:439–446
Perry JC, Da Cunha C, Anselmo-Franci J, Andreatini R, Miyoshi E, Tufik S, Vital MA (2004) Behavioural and neurochemical effects of phosphatidylserine in MPTP lesion of the substantia nigra of rats. Eur J Pharmacol 484:225–233
Proud CG (1992) Protein phosphorylation in translational control. Curr Top Cell Regul 32:243–369
Raught B, Gingras AC, Sonenberg N (2001) The target of rapamycin (TOR) proteins. Proc Natl Acad Sci USA 98:7037–7044
Reglodi D, Somogyvari-Vigh A, Vigh S, Maderdrut JL, Arimura A (2000) Neuroprotective effects of PACAP38 in a rat model of transient focal ischemia under various experimental conditions. Ann NY Acad Sci 921:119–128
Reglodi D, Tamas A, Somogyvari-Vigh A, Szanto Z, Kertes E, Lenard L, Arimura A, Lengvari I (2002) Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia. Peptides 23:2227–2234
Reglodi D, Tamas A, Lubics A, Szalontay L, Lengvari I (2004a) Morphological and functional effects of PACAP in 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Regul Pept 123:85–94
Reglodi D, Lubics A, Tamas A, Szalontay L, Lengvari I (2004b) Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson’s disease. Behav Brain Res 151:303–312
Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA (2002) Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci 22:10690–10698
Sawaguchi T (2000) The role of D1-dopamine receptors in working memory-guided movements mediated by frontal cortical areas. Parkinsonism Relat Disord 7:9–19
Schober A (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318:215–224
Schultz W (1984) Recent physiological and pathophysiological aspects of Parkinsonian movement disorders. Life Sci 34:2213–2223
Setlow B, McGaugh JL (2000) D2 dopamine receptor blockade immediately post-training enhances retention in hidden and visible platform versions of the water maze. Learn Mem 7:187–191
Shen YQ, Hebert G, Lin LY, Luo YL, Moze E, Li KS, Neveu PJ (2005) Interleukin-1beta and interleukin-6 levels in striatum and other brain structures after MPTP treatment: influence of behavioral lateralization. J Neuroimmunol 158:14–25
Stumm R, Kolodziej A, Prinz V, Endres M, Wu DF, Hollt V (2007) Pituitary adenylate cyclase-activating polypeptide is up-regulated in cortical pyramidal cells after focal ischemia and protects neurons from mild hypoxic/ischemic damage. J Neurochem 103:1666–1681
Takei N, Skoglosa Y, Lindholm D (1998) Neurotrophic and neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on mesencephalic dopaminergic neurons. J Neurosci Res 54:698–706
Tamas A, Lubics A, Lengvari I, Reglodi D (2006) Protective effects of PACAP in excitotoxic striatal lesion. Ann NY Acad Sci 1070:570–574
Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM (2002) A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA 99:467–472
Tanila H, Bjorklund M, Riekkinen P Jr (1998) Cognitive changes in mice following moderate MPTP exposure. Brain Res Bull 45:577–582
Tatton NA, Kish SJ (1997) In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience 77:1037–1048
Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H (2000a) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52:269–324
Vaudry D, Gonzalez BJ, Basille M, Pamantung TF, Fontaine M, Fournier A, Vaudry H (2000b) The neuroprotective effect of pituitary adenylate cyclase-activating polypeptide on cerebellar granule cells is mediated through inhibition of the CED3-related cysteine protease caspase-3/CPP32. Proc Natl Acad Sci USA 97:13390–13395
Vaudry D, Falluel-Morel A, Basille M, Pamantung TF, Fontaine M, Fournier A, Vaudry H, Gonzalez BJ (2003) Pituitary adenylate cyclase-activating polypeptide prevents C2-ceramide-induced apoptosis of cerebellar granule cells. J Neurosci Res 72:303–316
Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, Korsmeyer SJ, Przedborski S (2001) Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Proc Natl Acad Sci USA 98:2837–2842
Wang G, Qi C, Fan GH, Zhou HY, Chen SD (2005) PACAP protects neuronal differentiated PC12 cells against the neurotoxicity induced by a mitochondrial complex I inhibitor, rotenone. FEBS Lett 579:4005–4011
Wang G, Pan J, Tan YY, Sun XK, Zhang YF, Zhou HY, Ren RJ, Wang XJ, Chen SD (2008) Neuroprotective effects of PACAP27 in mice model of Parkinson’s disease involved in the modulation of K(ATP) subunits and D2 receptors in the striatum. Neuropeptides 42:267–276
Acknowledgments
This study was mainly supported by the French Ministry of Education and Research, with a grant to the Research Unit GREVIC, EA 3808 and by the Poitiers University Hospital. The authors wish to thank Tanguy Maurice for helpful discussions and Raymond Pontcharraud for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deguil, J., Chavant, F., Lafay-Chebassier, C. et al. Neuroprotective Effect of PACAP on Translational Control Alteration and Cognitive Decline in MPTP Parkinsonian Mice. Neurotox Res 17, 142–155 (2010). https://doi.org/10.1007/s12640-009-9091-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9091-4