Abstract
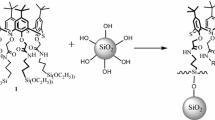
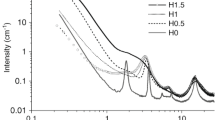
The effects of precursor structure and polycondensation conditions on the properties of hybrid nanoparticles synthesized from organo-trimethoxysilanes were studied. Hybrid nanoparticles containing groups capable of forming hydrogen bonds were synthesized from functional derivatives of 3-aminopropyltrimethoxysilane. For the synthesis of phenylurea-functionalized organosilica nanoparticles different approaches to nanoparticle preparation were used. It was shown that the nature of the functional groups (proton-donor or proton-acceptor) affects the aggregation of silica nanoparticles. Also, the difference in behavior of nanoparticles prepared using surface modification and polycondensation was demonstrated for different pH, ionic strength and solvent polarity. As a result, by changing the pH of the solutions, it is possible to shift the aggregation pattern of these nanoparticles, such as the size of the initially formed aggregates.
Similar content being viewed by others
References
Levy L, Sahoo Y, Kim K-S, Bergey EJ, Prasad PN (2002) Chem Mater 14:3715–3721
Wang Y, Forssberg E (2006) Int J Miner Process 81:1–14
Ulrich GD, Rieh JW (1982) J Colloid Interface Sci 87:257–265
Zhelev Z, Ohba H, Bakalova R (2006) J Am Chem Soc 128:6324–6325
Budny A, Novak F, Plumeré N, Schetter B, Speiser B, Straub D, Mayer HA, Reginek M (2006) Langmuir 22:10605–10611
Vertegel AA, Siegel RW, Dordick JS (2004) Langmuir 20:6800–6807
Banerjee S, Santra S (2009) Tetrahedron Lett 50:2037–2040
Smith JE, Wang L, Tan W (2006) Trends Anal Chem 25:848–855
Choi J, Burns AA, Williams RM, Zhou Z, Flesken-Nikitin A, Zipfel WR, Wiesner U, Nikitin AY (2007) J Biomed Opt 12:064007–1–064007–11
Caruso F, Caruso RA, Möhwald H (1998) Sci 282:1111–1114
Howard AG, Khdary NH (2005) Analyst 130:1432–1438
Bagwe RP, Hilliard LR, Tan W (2006) Langmuir 22:4357–4362
Bordes R, Tropsch J, Holmberg K (2010) Langmuir 26:3077–3083
Hermans TM, Broeren MAC, Gomopoulos N, Smeijers AF, Mezari B, Van Leeuwen ENM, Vos MRJ, Magusin PCMM, Hilbers PAJ, Van Genderen MHP, Sommerdijk NAJM, Fytas G, Meijer EW (2007) J Am Chem Soc 129:15631–15638
Kumar MNVR, Sameti M, Mohapatra SS, Kong X, Lockey RF, Bakowsky U, Lindenblatt G, Schmidt CH, Lehr C-M (2004) J Nanosci Nanotechnol 4:876–881
Hsieh Ch-Ch, Lin K-F (2005) J Mater Chem 15:4154–4160
Perrin DD, Armarego WLF (1988) Purification of laboratory chemicals, 3rd edn. Pergamon Press, Exeter
Pham KN, Fullston D, Sagoe-Crentsil K (2007) Aust J Chem 60:662–666
Gellermann C, Storch W, Wolter H (1997) J Sol-Gel Sci Technol 8:173–176
Stober W, Fink A, Bohn E (1968) J Colloid Interface Sci 26:62–69
Deng TS, Zhang QF, Zhang JY, Shen X, Zhu KT, Wu JL (2009) J Colloid Interface Sci 329:292–299
Lee Y-G, Park J-H, Oh C, Oh S-G, Kim YC (2007) Langmuir 23:10875–10878
Nakamura M, Ishimura K (2007) J Phys Chem C 111:18892–18898
Nakamura M, Ishimura K (2008) Langmuir 24:12228–12234
Nair BP, Pavithran C (2010) Langmuir 26(2):730–735
Davies G-L, Barry A, Gun’ko YK (2009) Chem Phys Lett 468:239–244
Jong MK (2009) Ceram Int 35:1015–1019
Mehendale NC, Lutz M, Spek AL, Klein Gebbink RJM, Koten G (2006) J Mol Catal A: Chem 257:167–175
Ziganshin MA, Yakimova LS, Khayarov KR, Gorbatchuk VV, Vysotsky MO, Böhmer V (2006) Chem Comm 3897–3899
Iler RK (1979) The chemistry of silica. Wiley-Interscience, New York
Huang H, Ruckenstein E (2006) Langmuir 22:4541–4546
Chakravarti N, Ghosh S, Dhar NR (1930) J Phys Chem 34:326–3
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gorbachuk, V.V., Yakimova, L.S., Mostovaya, O.A. et al. Silica Nanoparticles with Proton Donor and Proton Acceptor Groups: Synthesis and Aggregation. Silicon 3, 5–12 (2011). https://doi.org/10.1007/s12633-011-9077-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-011-9077-8