Abstract
Purpose
The objective of this study was to evaluate the exposure and the pharmacodynamic target attainment of piperacillin/tazobactam (PTZ) in adult critically ill patients.
Methods
We conducted a prospective observational study in the intensive care unit (ICU) of the Hôpital du Sacré-Cœur de Montréal (a Level I trauma centre in Montreal, QC, Canada) between January 2021 and June 2022. We included patients aged 18 yr or older admitted to the ICU who received PTZ by intravenous administration. Demographic and clinical characteristics were collected, and clinical scores were calculated. On study day 1 of antimicrobial therapy, three blood samples were collected at the following timepoints: one hour after PTZ dose administration and at the middle and at the end of the dosing interval. The sampling schedule was repeated on days 4 and 7 of therapy if possible. Samples were analyzed by ultra-high performance liquid chromatography with diode array detector to determine the total piperacillin concentration. Middle- and end-of-interval concentrations were used for target attainment analyses, and were defined as a concentration above the minimal inhibitory concentration of 16 mg·L−1, corresponding to the breakpoint of Enterobacteriaceae and Pseudomonas aeruginosa.
Results
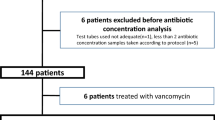
Forty-three patients were recruited and 202 blood samples were analyzed. The most prevalent dose was 3/0.375 g every six hours (n = 50/73 doses administered, 68%) with a 30-min infusion. We observed marked variability over the three sampling timepoints, and the median [interquartile range] piperacillin concentrations at peak, middle of interval, and end of interval were 109.4 [74.0–152.3], 59.3 [21.1–74.4], and 25.3 [6.8–44.6] mg·L−1, respectively. When assessing target attainment, 37% of patients did not reach the efficacy target of a trough concentration of 16 mg·L−1. The majority of patients who were underexposed were patients with normal to augmented renal clearance.
Conclusion
In this prospective observational study of adult ICU patients receiving intravenous PTZ, a large proportion had subtherapeutic concentrations of piperacillin. This was most notable in patients with normal to augmented renal clearance. More aggressive dosage regimens may be required for this subpopulation to ensure attainment of efficacy targets.
Résumé
Objectif
L’objectif de cette étude était d’évaluer l’exposition et l'atteinte des cibles pharmacodynamiques de la pipéracilline/tazobactam (PTZ) chez la patientèle adulte aux soins intensifs.
Méthodes
Nous avons réalisé une étude observationnelle prospective dans l'unité de soins intensifs (USI) de l'Hôpital du Sacré-Cœur de Montréal (un centre de traumatologie de niveau 1 à Montréal, QC, Canada) entre janvier 2021 et juin 2022. Nous avons inclus les patient·es adultes âgé·es de 18 ans ou plus admis·es à l'USI ayant reçu de la PTZ par administration intraveineuse. Les caractéristiques démographiques et cliniques ont été recueillies, et les scores cliniques ont été calculés. Au jour 1 de la thérapie antimicrobienne, trois échantillons sanguins ont été prélevés aux moments suivants : 1 h après l'administration de la dose de PTZ, au milieu et à la fin de l'intervalle d'administration. Le calendrier d’échantillonnage a été répété aux jours 4 et 7 de la thérapie si possible. Les échantillons ont été analysés par chromatographie liquide à ultra-haute performance avec détecteur à diodes pour déterminer la concentration totale de pipéracilline. Les concentrations du milieu et de fin d'intervalle ont été utilisées pour les analyses d'atteinte de cible, définie comme une concentration supérieure à la concentration minimale inhibitrice de 16 mg·L-1, associée aux Enterobacteriaceae et au Pseudomonas aeruginosa.
Résultats
Quarante-trois patient·es ont été recruté·es et 202 échantillons sanguins ont été analysés. La dose la plus prévalente était une dose de 3/0,375 g aux 6 h (n = 50/73 doses administrées, 68 %) avec une perfusion sur 30 min. Nous avons observé une variabilité marquée aux trois temps de prélèvement, et les concentrations médianes [intervalle interquartile] de pipéracilline au pic, au milieu et à la fin de l'intervalle étaient respectivement de 109,4 [74,0-152,3], 59,3 [21,1-74,4] et 25,3 [6,8-44,6] mg·L−1. Lors de l'évaluation de l'atteinte de la cible, 37 % des patient·es n'ont pas atteint la cible d'efficacité d'une concentration de 16 mg·L−1 à la fin de l’intervalle posologique. La majorité des patient·es sous-exposé·es étaient des personnes dont la clairance rénale était normale ou augmentée.
Conclusion
Dans cette étude observationnelle prospective de patient·es adultes aux soins intensifs recevant de la PTZ par voie intraveineuse, une grande proportion de patient·es présentait des concentrations sous-thérapeutiques de pipéracilline. Ceci était plus marqué chez les patient·es ayant une clairance rénale normale ou augmentée. Des schémas posologiques plus agressifs pourraient être nécessaires pour cette sous-population afin de favoriser l’atteinte des cibles d’efficacité.


Similar content being viewed by others
References
MacArthur RD, Miller M, Albertson T, et al. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin Infect Dis 2004; 38: 284–8. https://doi.org/10.1086/379825
Evans L, Rhodes A, Alhazzani W, et al. Executive summary: Surviving Sepsis Campaign: international guidelines for the management of sepsis and septic shock 2021. Crit Care Med 2021; 49: 1974–82. https://doi.org/10.1097/ccm.0000000000005357
Kollef MH, Shorr AF, Bassetti M, et al. Timing of antibiotic therapy in the ICU. Crit Care 2021; 25: 360. https://doi.org/10.1186/s13054-021-03787-z
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26: 1–12. https://doi.org/10.1086/516284
Abdul-Aziz MH, Alffenaar JW, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med 2020; 46: 1127–53. https://doi.org/10.1007/s00134-020-06050-1
Guilhaumou R, Benaboud S, Bennis Y, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit Care 2019; 23: 104. https://doi.org/10.1186/s13054-019-2378-9
Sumi CD, Heffernan AJ, Lipman J, Roberts JA, Sime FB. What antibiotic exposures are required to suppress the emergence of resistance for gram-negative bacteria? A systematic review. Clin Pharmacokinet 2019; 58: 1407–43. https://doi.org/10.1007/s40262-019-00791-z
El-Haffaf I, Caissy JA, Marsot A. Piperacillin-tazobactam in intensive care units: a review of population pharmacokinetic analyses. Clin Pharmacokinet 2021; 60: 855–75. https://doi.org/10.1007/s40262-021-01013-1
Fratoni AJ, Nicolau DP, Kuti JL. A guide to therapeutic drug monitoring of β-lactam antibiotics. Pharmacotherapy 2021; 41: 220–33. https://doi.org/10.1002/phar.2505
Chen IH, Nicolau DP. Augmented renal clearance and how to augment antibiotic dosing. Antibiotics (Basel) 2020; 9: 393. https://doi.org/10.3390/antibiotics9070393
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–29.
Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286: 1754–8. https://doi.org/10.1001/jama.286.14.1754
Marik PE, Taeb AM. SIRS, qSOFA and new sepsis definition. J Thorac Dis 2017; 9: 943–5. https://doi.org/10.21037/jtd.2017.03.125
Barletta JF, Mangram AJ, Byrne M, et al. Identifying augmented renal clearance in trauma patients: validation of the augmented renal clearance in trauma intensive care scoring system. J Trauma Acute Care Surg 2017; 82: 665–71. https://doi.org/10.1097/ta.0000000000001387
Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med 2001; 27: 859–64. https://doi.org/10.1007/s001340100909
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–10. https://doi.org/10.1001/jama.2016.0287
Legrand T, Vodovar D, Tournier N, Khoudour N, Hulin A. Simultaneous determination of eight β-lactam antibiotics, amoxicillin, cefazolin, cefepime, cefotaxime, ceftazidime, cloxacillin, oxacillin, and piperacillin, in human plasma by using ultra-high-performance liquid chromatography with ultraviolet detection. Antimicrob Agents Chemother 2016; 60: 4734–42. https://doi.org/10.1128/aac.00176-16
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 32nd edition; 2022. Available from URL: https://clsi.org/standards/products/elearning/education/using-m100-online-learning-performance-standards-for-antimicrobial-susceptibility-testing/ (accessed November 2023).
Zhanel GG, Adam HJ, Baxter MR, et al. 42936 pathogens from Canadian hospitals: 10 years of results (2007–16) from the CANWARD surveillance study. J Antimicrob Chemother 2019; 74: iv5–21. https://doi.org/10.1093/jac/dkz283
Beumier M, Casu GS, Hites M, et al. Elevated β-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol 2015; 81: 497–506.
Colman S, Stove V, De Waele JJ, Verstraete AG. Measuring unbound versus total piperacillin concentrations in plasma of critically ill patients: methodological issues and relevance. Ther Drug Monit 2019; 41: 325–30. https://doi.org/10.1097/ftd.0000000000000602
Schießer S, Hitzenbichler F, Kees MG, et al. Measurement of free plasma concentrations of beta-lactam antibiotics: an applicability study in intensive care unit patients. Ther Drug Monit 2021; 43: 264–70. https://doi.org/10.1097/ftd.0000000000000827
Briscoe SE, McWhinney BC, Lipman J, Roberts JA, Ungerer JP. A method for determining the free (unbound) concentration of ten beta-lactam antibiotics in human plasma using high performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci 2012; 907: 178–84. https://doi.org/10.1016/j.jchromb.2012.09.016
Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014; 58: 1072–83. https://doi.org/10.1093/cid/ciu027
Udy AA, Lipman J, Jarrett P, et al. Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit Care 2015; 19: 28. https://doi.org/10.1186/s13054-015-0750-y
Zander J, Döbbeler G, Nagel D, et al. Piperacillin concentration in relation to therapeutic range in critically ill patients—a prospective observational study. Crit Care 2016; 20: 79. https://doi.org/10.1186/s13054-016-1255-z
Smekal AK, Furebring M, Eliasson E, Lipcsey M. Low attainment to PK/PD-targets for β-lactams in a multi-center study on the first 72 h of treatment in ICU patients. Sci Rep 2022; 12: 21891. https://doi.org/10.1038/s41598-022-25967-9
Conil JM, Georges B, Mimoz O, et al. Influence of renal function on trough serum concentrations of piperacillin in intensive care unit patients. Intensive Care Med 2006; 32: 2063–6. https://doi.org/10.1007/s00134-006-0421-1
Carlier M, Carrette S, Roberts JA, et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit Care 2013; 17: R84. https://doi.org/10.1186/cc12705
Quinton MC, Bodeau S, Kontar L, et al. Neurotoxic concentration of piperacillin during continuous infusion in critically ill patients. Antimicrob Agents Chemother 2017; 61: e00654–17. https://doi.org/10.1128/aac.00654-17
Imani S, Buscher H, Marriott D, Gentili S, Sandaradura I. Too much of a good thing: a retrospective study of β-lactam concentration–toxicity relationships. J Antimicrob Chemother 2017; 72: 2891–7. https://doi.org/10.1093/jac/dkx209
Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 2014; 77: 3–11. https://doi.org/10.1016/j.addr.2014.07.006
Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 2009; 37: 840–51. https://doi.org/10.1097/ccm.0b013e3181961bff
Brunetti L, Poustchi S, Cunningham D, et al. Clinical and economic impact of empirical extended-infusion piperacillin-tazobactam in a community medical center. Ann Pharmacother 2015; 49: 754–60. https://doi.org/10.1177/1060028015579427
Chan AJ, Lebovic G, Wan M, et al. Impact of extended-infusion piperacillin-tazobactam in a Canadian community hospital. Infect Med 2023; 2: 31–5. https://doi.org/10.1016/j.imj.2023.01.005
Fawaz S, Barton S, Nabhani-Gebara S. Comparing clinical outcomes of piperacillin-tazobactam administration and dosage strategies in critically ill adult patients: a systematic review and meta-analysis. BMC Infect Dis 2020; 20: 430. https://doi.org/10.1186/s12879-020-05149-6
Wong G, Briscoe S, Adnan S, et al. Protein binding of β-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother 2013; 57: 6165–70. https://doi.org/10.1128/aac.00951-13
Al-Shaer MH, Alghamdi WA, Graham E, Peloquin CA. Meropenem, cefepime, and piperacillin protein binding in patient samples. Ther Drug Monit 2020; 42: 129–32. https://doi.org/10.1097/ftd.0000000000000675
El-Haffaf I, Guilhaumou R, Velly L, Marsot A. Impact of piperacillin unbound fraction variability on dosing recommendations in critically ill patients. Br J Clin Pharmacol 2023; 89: 1502–8. https://doi.org/10.1111/bcp.15619
Nicasio AM, VanScoy BD, Mendes RE, et al. Pharmacokinetics-pharmacodynamics of tazobactam in combination with piperacillin in an in vitro infection model. Antimicrob Agents Chemother 2016; 60: 2075–80. https://doi.org/10.1128/aac.02747-15
Kalaria SN, Gopalakrishnan M, Heil EL. A population pharmacokinetics and pharmacodynamic approach to optimize tazobactam activity in critically ill patients. Antimicrob Agents Chemother 2020; 64: e02093–19. https://doi.org/10.1128/aac.02093-19
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
Amélie Marsot, David Williamson, Martin Albert, Marc-André Smith, and Ibrahim El-Haffaf contributed to study conception and design. David Williamson, Djamila Hachemi, Thomas Pesout, and Virginie Williams collected and provided the clinical data. Ibrahim El-Haffaf quantified blood samples and analyzed the data. Ibrahim El-Haffaf, Amélie Marsot, and David Williamson prepared the manuscript draft. Amélie Marsot and David Williamson supervised the work.
Disclosures
The authors declare no conflicts of interest.
Funding statement
This project has received funding from the Canadian Society of Hospital Pharmacists and from the Fonds Servier granted by the Faculty of Pharmacy of Université de Montréal. Amélie Marsot acknowledges support from the Fonds de Recherche du Québec-Santé (FRQS) Research Scholars—Junior 1 (Young Researcher Establishment) Career Scholarship. David Williamson acknowledges support from the FRQS Clinical Research Scholars – Junior 2. Ibrahim El-Haffaf acknowledges receiving a scholarship from the Faculty of Pharmacy of Université de Montréal and from the FRQS (Doctoral Training Scholarship). The funders had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, the preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
Prior conference presentations
Data reported in this manuscript were presented in part in the 33rd European Congress of Clinical Microbiology & Infectious Diseases (15–18 April 2023, Copenhagen, Denmark) and in the 90th edition of the Congrès de l’Acfas (8–12 May 2023, Montreal, QC, Canada).
Editorial responsibility
This submission was handled by Dr. Alexis F. Turgeon, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Haffaf, I., Marsot, A., Hachemi, D. et al. Exposure levels and target attainment of piperacillin/tazobactam in adult patients admitted to the intensive care unit: a prospective observational study. Can J Anesth/J Can Anesth 71, 511–522 (2024). https://doi.org/10.1007/s12630-023-02689-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02689-8