Abstract
Purpose
Deceased organ donation is predicated on timely identification and referral (IDR) of potential organ donors. Many Canadian provinces have legislated mandatory referral of potential deceased donors. Untimely or missed IDRs are safety events where best or expected practice has not occurred causing preventable harm to patients and denying families the opportunity of donation at end of life (EOL) as well as denying transplant waitlist patients access to lifesaving organs.
Methods
We requested donor definitions and data to calculate IDR, consent, and approach rates from all Canadian organ donation organizations (ODOs) for 2016–2018. We then estimated the number of missed IDR patients who were eligible for approach (safety events) and the associated preventable harm to patients at EOL and on transplant waitlists.
Results
Annually, there were 63–76 missed IDR patients eligible for approach (3.6–4.5 per million population [PMP]) from four ODOs—three with mandatory referral legislation. Applying each ODO’s approach and consent rates for the corresponding year, there were 37–41 missed donors (2.4 donor PMP) annually. Assuming three transplants per donor, the theoretical number of missed transplants would be 111–123 (6.4–7.3 transplants PMP) annually.
Conclusions
Data from four Canadian ODOs show that missed IDR safety events resulted in important preventable harm measured by a lost opportunity for donation of 2.4 donors PMP annually and 354 potentially missed transplants between 2016 and 2018. Given that 223 patients died on Canada’s waitlist in 2018, national donor audits and quality improvement initiatives to optimize IDR are essential to reduce preventable harm to these vulnerable populations.
Résumé
Objectif
Le don d’organes provenant de personnes décédées repose sur l’identification et l’aiguillage en temps opportun des donneurs d’organes potentiels. De nombreuses provinces canadiennes ont légiféré sur l’aiguillage obligatoire des donneurs potentiels décédés. Les identifications et aiguillages inopportuns ou manqués constituent des événements liés à la sécurité pour lesquels la meilleure pratique ou la pratique attendue n’a pas eu lieu, causant des préjudices évitables aux patients et privant les familles de la possibilité de faire un don en fin de vie, tout en refusant aux patients inscrits sur une liste d’attente de greffe un accès à des organes vitaux.
Méthode
Nous avons demandé les définitions et les données sur les donneurs pour calculer les taux d’identification et d’aiguillage, de consentement et d’approche de tous les organismes canadiens de don d’organes (ODO) pour la période de 2016-2018. Nous avons ensuite estimé le nombre de patients n’ayant pas été identifiés et aiguillés mais qui étaient admis à être approchés (événements liés à la sécurité) et les préjudices évitables aux patients en fin de vie et sur les listes d’attente pour une greffe.
Résultats
Chaque année, l’identification et l’aiguillage a échoué pour 63 à 76 patients éligibles (3,6 à 4,5 par million d’habitants [PMH]) dans quatre ODO – dont trois possédant une législation rendant l’aiguillage obligatoire. En appliquant l’approche et les taux de consentement de chaque ODO pour l’année correspondante, on a constaté qu’il y avait de 37 à 41 donneurs manqués (2,4 donneurs PMH) chaque année. En supposant trois greffes par donneur, le nombre théorique de greffes manquées serait de 111 à 123 (6,4 à 7,3 greffes PMH) par an.
Conclusion
Les données de quatre ODO canadiens montrent que les événements de sécurité liés à une identification et un aiguillage manqués ont entraîné d’importants préjudices évitables, mesurés par une occasion perdue de donner pour 2,4 donneurs PMH chaque année et 354 greffes potentiellement manquées entre 2016 et 2018. Étant donné que 223 patients sont décédés sur la liste d’attente du Canada en 2018, les vérifications nationales des donneurs et les initiatives d’amélioration de la qualité visant à optimiser l’identification et l’aiguillage sont essentielles pour réduire les préjudices évitables causés à ces populations vulnérables.
Similar content being viewed by others
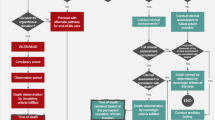
Despite sustained improvements in deceased organ donation rates in Canada, the gap between patients on the transplant waitlist and available organs persists.1 As a result, patients continue to die on, or be withdrawn due to deteriorating health, from the transplant waitlist.2 Deceased organ donation is predicated on timely identification and referral (IDR) of potential organ donors, as failure to perform this first, critical step jeopardizes the downstream donation process (Figure).3,4,5 Furthermore, in Canada, five provinces (BC, MB, ON, NS, AB) have legislated mandatory referral/consideration of deceased donors to ensure IDR. In addition to untimely or missed IDR, missed donation opportunities (MDO) can occur at every step along the donation pathway. Missed donation opportunities are safety events where best or expected practice has not occurred.
Sequence of care in deceased donation. Reproduced and adapted with permission from Figure 2: Sequence of care in deceased donation in relation to notification and referral. Zavalkoff S, Shemie SD, Grimshaw JM, et al. Potential organ donor identification and system accountability: expert guidance from a Canadian consensus conference. Can J Anesth 2019; 66: 432–47. https://doi.org/10.1007/s12630-018-1252-6
The 2016 Potential Organ Donor Identification and System Accountability expert guidance advanced missed IDR as a preventable harm to both patients on the transplant list, who are denied access to lifesaving organs, and to families and patients at the end of life (EOL), who are denied the opportunity to donate.3 Currently, the Canadian organ donation and transplantation (ODT) system is unable to quantify this preventable harm, nor plan and monitor improvement initiatives to reduce it. Our objective was to determine the national rate of donor IDR, estimate the number of MDO from missed IDR, and quantify the consequential preventable harm to Canadians patients and their families at EOL and on the transplant waitlist.
Methods
The Research Institute of the McGill University Health Centre, in collaboration with Canadian Blood Services, consulted with all eleven Canadian provincial organ donation organizations (ODOs) to complete the study. The study was granted an exemption letter for ethics review by The McGill University Health Centre Research Ethics Board as a quality improvement project.
Survey and interview development
The survey and interview guide were designed using the methodology of Burns et al.6 The research team first generated an exhaustive list of themes based on the existing literature. Themes were then drafted as survey and interview questions and divided into domains of potential donor audit practices, procedures and resources, and operational definitions. Potential survey and interview questions were then circulated to expert consultants (donor coordinators and donor physicians) for feedback. The research team and an experienced deceased donor coordinator reduced the list of potential survey questions to a target of 25 or fewer.7 Our team then determined whether each item should be 1) retained, 2) retained with edits, 3) excluded, or 4) retained and moved to the interview guide.
Prior to data collection, two donor coordinators provided feedback on ease of survey completion, flow, clarity, relevance, completeness, face validity, content validity, redundancy, and time for completion. We revised the survey based on the provided feedback. The survey was created and hosted on the Interceptum (Acquiro Systems, Inc., Gatineau, QC, Canada) platform. The final survey had 21 questions.
Survey administration
Each ODO was invited to participate in the study as part of a larger environmental scan. A member of the research team established a point of contact and introduced the project. Subsequently, each ODO was emailed a cover letter with a link to an electronic survey. Up to three weekly reminders were sent after the initial invitation.
Interviews
After survey submission, the research coordinator booked a one-hour telephone interview with each ODO representative to clarify and complete submitted survey responses. Interviews were recorded for transcription with verbal consent. Data were entered and summarized in a spreadsheet. No patient level data were collected, nor did we collect information about tissue donation.
The structured interview guide included questions about donor audit objectives, frequency, scope (hospital sites and patient inclusion and exclusion criteria), methodology of data collection, human and other resources required to conduct donor audits (including training, standard operating procedures, policies), and outcomes reporting and feedback processes including accountability structures. In addition, we sought to collect any missing data from the survey.
We collected ODO operational definitions for the following terms: potential, identified, referred, missed referral, eligible, approached, consented, and nonutilized donors. We obtained available aggregate rates for IDR, consent, and approach from the calendar years 2016, 2017, and 2018. These years were chosen as this research was initiated in 2019 but was delayed due to the COVID-19 pandemic. We accepted each ODO’s local definition of a potential, eligible, approached, and consented donor. We also asked each ODO to describe how they calculated these rates based on their operational definitions and assessed whether the calculation aligned with the method outlined in Table 1. When it did not, we collaborated with the ODO to complete the calculations using their raw data. If we could not harmonize ODO data with calculations in Table 1, the data were not included. In the absence of national agreed upon definitions and metrics, we based Table 1 on work developed by the Canadian Deceased Donation Data Working Group.8
Calculation of preventable harm
We considered preventable harm to both potential organ donors and their families (i.e., depriving the opportunity to donate) and to patients on the transplant waitlist (i.e., depriving access to a lifesaving/life-enhancing transplant). Missed IDR patients are those who met the ODO trigger for referral but were not referred. Missed eligible IDR patients are missed IDR patients whom the ODO deemed suitable for approach (Table 1). We calculated the mean IDR, approach, and consent rates along with the 95% confidence interval. To estimate preventable harm, we applied the ODO’s annual proportion of donors who were eligible for approach (for referred donors) to the ODO’s number of nonreferred donors (missed IDR). Two ODOs directly provided the proportion of referred patients who were eligible for approach. For the other two ODOs, we used the denominator of their approach rate as a surrogate for the number of potential donors who were medically eligible for approach. The other seven ODOs were unable to provide sufficient data to estimate the metrics of interest and were not included in the analysis.
We considered missed eligible IDR as patients who were harmed at EOL, as they were denied the opportunity to consider donation by not being referred. Next, we applied the ODO’s actual approach and consent rates for the corresponding year, and used an average of three organs per donor,1 to estimate the number of missed transplants. While a deceased organ donor may provide up to eight organs, three is the average number a donor in Canada provides.1 This final number of missed transplants was then equated with the number of patients on the transplant waitlist who were harmed by missed IDR. When applied to the provincial population of the four ODOs included in this analysis, we calculated this as missed referrals per million population (PMP).9,10 The denominator of PMP is the traditional metric for deceased donation, living donation, and transplantation rates and which allow interjurisdictional comparison. Analysis was carried out with Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and Stata (StataCorp, College Station, TX, USA).
Results
Three ODOs provided definitions and metrics that aligned with those defined in Table 1. One ODO’s raw data allowed the necessary data to be sourced. Together, these four ODOs provided data that could be harmonized to calculate mean IDR, approach, and consent rates for 2016–2018 (Table 2). Both potential donors after neurologic and circulatory determination of death were included. Data were de-identified to maintain each ODO’s confidentiality.
Annually, there were 75–105 missed IDR safety events in four provinces where patients who met the ODO’s clinical referral triggers were not referred. Of these, 63–76 were theoretically eligible to be approached for donation (3.6–4.5 PMP),10 and having not been IDR, suffered preventable harm at the EOL by not being offered the opportunity to donate. By applying each ODO’s actual approach and consent rates for the corresponding year, the theoretical number of missed consented donors was 37–41 (2.4 donor PMP). The corresponding theoretical number of missed transplants was 111–123 annually (6.4–7.3 transplants PMP), and assuming three transplants per donor,1 this represents preventable harm to a total of 354 waitlisted transplant patients between 2016 and 2018 (Table 3).
Interpretation
We requested data on donation metrics from all Canadian ODOs. Given the lack of national consistency in conducting donor audits, only four ODOs could provide data to estimate preventable harm. Annually, 63–76 missed eligible IDR patients were harmed at the EOL by being denied the opportunity to donate. There were 37–41 MDO (2.4 donors PMP annually) due to missed IDR once the ODO’s actual approach and consent rates were applied. This resulted in an estimated 111–123 patients on the transplant waitlist potentially harmed each year of the study by being denied a lifesaving or life-enhancing transplant (6.4–7.3 transplants PMP).
These estimations of harm are significant when considered in the context of Canada’s deceased ODT system metrics. In 2018, 4,351 patients were on the transplant waitlist and 223 patients died waiting. Given the rare scenario that allows for organ donation, in addition to these figures, the reported MDO from missed IDR are substantial. In 2018, there were 20.3 donors PMP,1 so 2.4 missed donors PMP represents an unrealized increase of 12%. In addition, 7.3 missed transplants PMP represents a nearly 10% unrealized increase from the 2018 rate of 76.3 transplants PMP.1 The potential harm of missed IDR is likely more significant than estimated, given calculations were only based on four ODO.
Missed and untimely IDR has been described previously in Canada and internationally. The ACCORD project found that 35% of patients in Europe who died of devastating brain injury were never referred.11 The Alberta death audit found that 18% of brain death cases were not referred.12 Timely referrals entail calling the ODO when EOL conversations are planned and/or conducted to ensure sufficient time for the donation process. Untimely referral compromises the donation process by not allowing time for donor assessment, mobilizing resources, and a well-planned approach. Krmpotic et al. reported only 66% of medically suitable potential donors in Ontario had timely referrals before withdrawal of life-sustaining measures (WLSM), resulting in 251 MDOs over two years.13 Singh et al. found the most common reason for not approaching a patient with donor potential in Ontario was referrals made at the time of or after WLSM.14 Organ donation organization A in our study showed an improved IDR rate and a decline in approach rate for the three years reviewed, likely explained by untimely referrals. Missed and untimely referrals of potential donors are particularly problematic from the emergency department (ED). In the UK, Empson et al. described that 53% of potential donors failed to be referred even though 16% of these were on the donation registry.15 A systematic review showed that up to 86% of patients after neurologic determination of death and 75% of donors after circulatory determination of death were not referred from the ED.16 Finally, an Ontario ED donor audit found ten postmortem referrals that potentially would have increased the hospital’s donation rate, had they been referred.17 While the problem of IDR has been broadly described, our study is unique in quantifying its downstream preventable harm as a safety event.
Traditionally, we recognize safety events that cause direct harm to patients like a medication error or wrong-site surgery. In many jurisdictions, we are obligated to disclose such events to the patients.18 When a potential organ donor is not IDR this causes downstream, but disconnected, harm to a vulnerable patient awaiting transplant. There is no official accountability for this safety event; no disclosure is required. Offering donation is recommended as a standard part of high quality EOL care.3,5,19,20,21,22 Moreover, it is essential we respect people’s dying wishes which they may have registered or expressed to family. When standards for management of sepsis or myocardial infarction are not respected in clinical care, these are considered safety events. The same approach currently does not apply to guidelines advising routine and timely IDR of potential deceased organ donors.
The lack of available data from ODOs in our study is itself an important finding. The fact that only four of eleven ODOs could provide the requested data highlights Canada’s need for a national, standardized approach to donor audits including definitions, a minimal data set, and metrics. This would allow measurement and reporting of the disconnected, downstream harm of MDOs including missed or untimely donor IDR. It would also facilitate goal setting, planning, and evaluation of quality improvement interventions.5
We acknowledge there are limitations to our study. While the lack of available data from all ODOs highlights a weakness of the Canadian ODT system, it also represents a limitation of this work. It is likely that the unmeasured harm from missed IDR is greater than reported, as our conclusions are only based on data from four ODOs. While the approach and consent rates are based on the ODOs actual rates during those years, we have made assumptions about the utilization rates and the number of organs each donor would yield. Since not all theoretically consented donors may actualize, this is a source of potential overestimation. Furthermore, ODO definitions and reporting metrics were misaligned. For example, the definition of approach (e.g., inclusive of health care provider approach vs ODO-only approach) varied by ODO, and thus the approach rate may differ. Referral rates may be impacted by a lack of standard referral criteria across jurisdictions. We attempted to mitigate these limitations by clarifying our understanding of these nuances through telephone interviews and by sourcing raw ODO data when required. In addition, data presented are a combination of both neurologic and circulatory determination of death which does not allow us to comment on specific referral patterns by donation type. We also did not weigh our results by provincial population size.
Conclusions
Missed identification and referral of potential organ donors causes harm by denying both the opportunity to donate at end of life and access to lifesaving or life-enhancing organs to vulnerable patients awaiting transplant. This safety event and its consequential preventable harm is unmeasured and unrecognized, so there is no accountability or disclosure of these patient safety events. Future work is needed to standardize the definition of a potential donor, clinical referral triggers, and the reporting of missed donor identification and referral to allow for accurate measurement and reporting of these patient safety events and to facilitate accountability mechanisms.
References
Canadian Blood Services. Organ and tissue donation and transplantation: system progress report 2018. Available from URL: https://professionaleducation.blood.ca/sites/default/files/organ_and_tissue_donation_and_transplantation_-_system_progress_report_2018.pdf (accessed November 2022).
Canadian Blood Services. Organ donation and transplantation in Canada: system progress report 2006–2015. Available from URL: https://profedu.blood.ca/sites/default/files/odt_report.pdf (accessed November 2022).
Zavalkoff S, Shemie SD, Grimshaw JM, et al. Potential organ donor identification and system accountability: expert guidance from a Canadian consensus conference. Can J Anesth 2019; 66: 432–47. https://doi.org/10.1007/s12630-018-1252-6
Domínguez-Gil B, Delmonico FL, Shaheen FA, et al. The critical pathway for deceased donation: reportable uniformity in the approach to deceased donation. Transpl Int 2011; 24: 373–8. https://doi.org/10.1111/j.1432-2277.2011.01243.x
Domínguez-Gil B, Murphy P, Procaccio F. Ten changes that could improve organ donation in the intensive care unit. Intensive Care Med 2016; 42: 264–7. https://doi.org/10.1007/s00134-015-3833-y
Burns KE, Duffett M, Kho ME, et al. A guide for the design and conduct of self-administered surveys of clinicians. CMAJ 2008; 179: 245–52. https://doi.org/10.1503/cmaj.080372
Passmore C, Dobbie AE, Parchman M, Tysinger J. Guidelines for constructing a survey. Fam Med 2002; 34: 281–6.
Hornby K, Shemie SD, Appleby A, et al. Development of a national minimum data set to monitor deceased organ donation performance in Canada. Can J Anesth 2019; 66: 422–31. https://doi.org/10.1007/s12630-018-01290-8
Canadian Institute for Health Information. Treatment of End-Stage Organ Failure in Canada, Canadian Organ Replacement Register, 2010 to 2019: Donors — Data Tables. Ottawa: CIHI; 2021.
Statistics Canada. Estimates of population (2016 Census and administrative data), by age group and sex for July 1st, Canada, provinces, territories, health regions (2018 boundaries) and peer groups. Available from URL: https://doi.org/10.25318/1710013401-eng (accessed November 2022).
Accord. Work package 5 – increasing the collaboration between donor transplant coordinators and intensive care professionals, 2015. Available from URL: http://www.accord-ja.eu/sites/default/files/download_documents/ACCORD_WP_5_ICU_%26_DTC_Collaboration_FINAL_REPORT.pdf (accessed November 2022).
Kramer AH, Hornby K, Doig CJ, et al. Deceased organ donation potential in Canada: a review of consecutive deaths in Alberta. Can J Anesth 2019; 66: 1347–55. https://doi.org/10.1007/s12630-019-01437-1
Krmpotic K, Payne C, Isenor C, Dhanani S. Delayed referral results in missed opportunities for organ donation after circulatory death. Crit Care Med 2017; 45: 989–92. https://doi.org/10.1097/ccm.0000000000002432
Singh JM, Ball IM, Hartwick M, et al. Factors associated with consent for organ donation: a retrospective population-based study. CMAJ 2021; 193: E1725–32. https://doi.org/10.1503/cmaj.210836
Empson K BS, Kerr J, Gardiner D. Organ donation in emergency departments: an analysis of best practice. Emerg Med 2017; 34: A877–8. https://doi.org/10.1136/emermed-2017-207308.25
McCallum J, Ellis B, Dhanani S, Stiell IG. Solid organ donation from the emergency department - a systematic review. CJEM 2019; 21: 626–37. https://doi.org/10.1017/cem.2019.365
McCallum J, Yip R, Dhanani S, Stiell I. Solid organ donation from the emergency department–missed donor opportunities. CJEM 2020; 22: 701–7. https://doi.org/10.1017/cem.2019.482
Canadian Patient Safety Institute. Canadian disclosure guidelines: being open and honest with patients and families, 2011. Available from URL: https://www.patientsafetyinstitute.ca/en/toolsResources/disclosure/Documents/CPSI%20Canadian%20Disclosure%20Guidelines.pdf (accessed November 2022).
Shemie SD, Robertson A, Beitel J, et al. End-of-life conversations with families of potential donors: leading practices in offering the opportunity for organ donation. Transplantation 2017; 101: S17–26. https://doi.org/10.1097/tp.0000000000001696
The Transplantation Society: World Health Organization. The Madrid Resolution on organ donation and transplantation: national responsibility in meeting the needs of patients, guided by the WHO principles. Transplantation 2011; 91: S29–31. https://doi.org/10.1097/01.tp.0000399131.74618.a5
The Canadian Association of Critical Care Nurses. Position statement: deceased organ and tissue donation, 2015. Available from URL: https://caccn.ca/wp-content/uploads/2019/12/PS112019DOTD.pdf (accessed November 2022).
Shemie SD. Learning from a social experiment in consent for deceased organ donation. CMAJ 2021; 193: E1735–6. https://doi.org/10.1503/cmaj.190761
Author contributions
Samara Zavalkoff, Shauna O’Donnell, and Jehan Lalani contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Isabela Fabri Karam contributed to the acquisition and analysis of data. Sam Shemie and Lee James contributed to the interpretation of data.
Acknowledgements
Ms. Carrie Benninger and Ms. Heather Cohrs for reviewing the survey data collection form. Kathleen Sullivan at Canadian Blood Services for setting up and managing the electronic survey. We thank all Canadian ODOs for their collaboration and participation in this study namely Kiran Khatar, Andreas Kramer, Deanna Paulson, Jaime Robin Partyka, Betty Wolfe, Nancy Dodd, Janice Beitel, Hugues Villeneuve, Janet Gallant, Rita Berry, Nadya Savoie, Angela Carpenter, and Kim Parsons.
Disclosures
Dr. Zavalkoff disclosed funding from Canadian Blood Services and the Organ Donation and Transplant Collaborative. Dr. Shemie disclosed that he is a medical advisor for deceased organ donation at Canadian Blood Services, and he disclosed government work. Ms. O’Donnell and Ms. Fabri Karam disclosed receiving partial salary compensation from the Canadian Blood Services grant awarded to Dr. Zavalkoff to conduct the research activities related to this manuscript and the Organ Donation and Transplant Collaborative. Ms. Lalani and Ms. James are employed by Canadian Blood Services.
Funding statement
The work was supported by a grant from Canadian Blood Services and the Organ Donation and Transplantation Collaborative. Canadian Blood Services receives funding from the provincial and territorial Ministries of Health and the federal government, through Health Canada. The views expressed herein do not necessarily represent the views of the federal, provincial, or territorial governments. Canadian Blood Services is a national, not‐for-profit charitable organization. In the domain of organ donation and transplantation, it provides national services in the development of leading practices, system performance measurement, interprovincial sharing registries, and public awareness and education. The Organ Donation and Transplantation Collaborative is an initiative led by Health Canada with provinces’ and territories’ health officials (except Quebec), Canadian Blood Services, patients, families, clinicians, and researchers across Canada. The vision of the Collaborative is to facilitate collaboration on an organ donation and transplantation ecosystem that results in better patient outcomes and an increase in the number and quality of successful transplantations.
Prior conference presentations
Preliminary results from the environmental scan were presented by Dr. Zavalkoff at the Canadian Critical Care Forum held in Toronto, ON, Canada on 11 November 2019 in a presentation entitled, “Donor identification and referral: preventable death and disability.” No abstract was published and no written material was disseminated to forum attendees.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zavalkoff, S., O’Donnell, S., Lalani, J. et al. Preventable harm in the Canadian organ donation and transplantation system: a descriptive study of missed organ donor identification and referral. Can J Anesth/J Can Anesth 70, 886–892 (2023). https://doi.org/10.1007/s12630-023-02399-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02399-1