Abstract
Purpose
The purpose of this study was to evaluate the effects of preoperative forced-air warming on intraoperative hypothermia.
Methods
In this randomized-controlled trial, adult patients scheduled for elective, non-cardiac surgery under general anesthesia were stratified by scheduled surgical duration (< 2.5 hr or ≥ 2.5 hr) and then randomized to a pre-warming group using a BairPaws™ forced-air warming system for at least 30 min preoperatively or to a control group with warmed blankets on request. All patients were warmed intraoperatively via convective forced-air warming blankets. Perioperative temperature was measured using the SpotOn™ temperature system consisting of a single-use disposable sensor applied to the participant’s forehead. The primary outcome was the magnitude of intraoperative hypothermia calculated as the area under the time-temperature curve for core temperatures < 36°C between induction of general anesthesia and leaving the operating room. Secondary outcomes included surgical site infections, packed red blood cell requirements, and 24 hr postoperative opioid consumption.
Results
Two hundred participants were analyzed (101 control; 99 pre-warmed). Pre-warmed participants had a lower median [interquartile range] magnitude of hypothermia than controls (0.00 [0.00-0.12] °C·hr−1 vs 0.05 [0.00-0.36] °C·hr−1, respectively; median difference, −0.01°C·hr−1; 95% confidence interval, −0.04 to 0.00°C·hr−1; P = 0.005). There were no between-group differences in the secondary outcomes.
Conclusion
A minimum of 30 min of preoperative forced-air convective warming decreased the overall intraoperative hypothermic exposure. While redistribution hypothermia still occurs despite pre- and intraoperative forced-air warming, their combined application results in greater preservation of intraoperative normothermia compared with intraoperative forced-air warming alone.
Trial registration
www.clinicaltrials.gov (NCT02177903). Registered 25 June 2014.
Résumé
Objectif
L’objectif de cette étude était d’évaluer les conséquences du réchauffement préopératoire par air forcé sur l’hypothermie peropératoire.
Méthodes
Dans cette étude randomisée contrôlée, des patients adultes devant subir une chirurgie élective non cardiaque sous anesthésie générale ont été stratifiés en fonction de la durée prévue de l’intervention (< 2,5 h ou ≥ 2,5 h) puis randomisés dans un groupe de réchauffement préalable utilisant un système à air forcé BairPaws™ pendant au moins 30 min en préopératoire ou dans un groupe témoin avec couvertures chauffantes sur demande. Tous les patients ont été réchauffés pendant l’intervention au moyen de couvertures chauffantes à air forcé par convection. La température périopératoire a été mesurée au moyen du système de surveillance de la température SpotOnMD consistant en un capteur jetable à usage unique placé sur le front des patients. Le critère principal d’évaluation était l’ampleur de l’hypothermie peropératoire calculée comme l’aire sous la courbe température-temps pour des températures centrales < 36 °C entre l’induction de l’anesthésie générale et la sortie de la salle d’opération. Les critères d’évaluation secondaires ont inclus les infections du site chirurgical, le nombre de culots de globules rouges nécessaires et la consommation d’opioïdes au cours des 24 heures postopératoires.
Résultats
Les résultats de 200 participants ont été analysés (101 contrôles; 99 patients préréchauffés). L’amplitude médiane de l’hypothermie [écart interquartile] des participants préréchauffés a été plus faible que celle des contrôles (respectivement, 0,00 [0,00-0,12] °C·hr−1 contre 0,05 [0,00-0,36] °C·hr−1; différence entre médianes, −0,01 °C·hr−1; intervalle de confiance à 95%, −0,04 à 0,00 °C·hr−1; P < 0,005). Il n’y a pas eu de différence entre les groupes sur les critères d’évaluation secondaires.
Conclusion
Un minimum de 30 min de réchauffement préopératoire au moyen d’un système à air forcé à convection a diminué l’exposition globale à l’hypothermie peropératoire. Bien qu’une hypothermie de redistribution survienne toujours en dépit du réchauffement peropératoire à l’air forcé, leur application combinée entraîne une meilleure conservation de la normothermie peropératoire, comparativement au réchauffement peropératoire à l’air forcé seul.
Enregistrement de l’essai clinique
www.essaiscliniques.gov (NCT02177903). Enregistré le 25 juin 2014.
Similar content being viewed by others
Hypothermia, generally defined as a core body temperature < 36°C, can occur at any stage in the perioperative period.1 Hypothermia should be avoided as it has been associated with an increased risk of adverse outcomes including surgical site infections (SSIs), coagulopathy, and myocardial complications.1,2,3,4,5 It also impacts patient recovery, prolonging recovery from anesthesia6 and increasing hospital length of stay (LOS).1,7
With the induction of general anesthesia, thermal regulation is significantly impaired as the loss of intrinsic compensatory mechanisms compounded by anesthetic-induced vasodilation leads to the redistribution of body heat from the core to peripheral tissues.8,9 Perioperative hypothermia is consequently observed in as many as two-thirds of patients despite the use of intraoperative warming techniques, the most common being convective forced-air warming.10 While intraoperative forced-air warming can eventually restore normothermia, it is inadequate for shorter procedures because of the insufficient rewarming time; thus, the consequences of this period of hypothermia are uncertain.11,12,13
Preoperative forced-air warming (i.e., pre-warming) reduces the potential for heat loss that occurs during post-induction redistribution by cutaneously transferring heat to peripheral tissues, thereby decreasing the core-to-periphery temperature gradient and in turn reducing the overall incidence of hypothermia.11,14 Inconsistency in the adoption of preoperative temperature management into clinical practice remains because of the uncertain impact of reducing redistribution hypothermia15 and the impracticality of using the most widely reported pre-warming duration of 60 min16,17 in a busy operating room environment. Improved characterization of the effects of pre-warming on intraoperative hypothermic exposure to identify the magnitude of hypothermia for a wide variety of surgical procedures may assist in understanding the extent to which different hypothermic patterns contribute to clinically important outcomes and how they may be impacted by pre-warming.
Accordingly, this randomized-controlled trial evaluated the effects of pre-warming using BairPaws™ (3M Canada, London, ON, Canada) convective forced-air warming gowns on intraoperative hypothermia in adult patients undergoing general anesthesia for elective non-cardiac surgery. We tested the primary hypothesis that a minimum 30-min period of pre-warming would reduce the magnitude of intraoperative hypothermic exposure as determined by the area under the time-temperature curve (AUC) for a core temperature < 36.0°C between induction of general anesthesia and leaving the operating room. We also tested the secondary hypotheses that pre-warming would improve patient anxiety, improve thermal comfort, and reduce the incidence of SSIs, estimated blood loss, red blood cell transfusions, flannel blanket use, postoperative opioid consumption, and postanesthetic care unit (PACU) and hospital LOS.
Methods
With research ethics board (FHREB 2014-02; June 2014) approval, this randomized-controlled trial enrolled participants from September 9, 2014 to December 2, 2015 with follow-up ending on March 17, 2016. With written informed consent, we planned to recruit 200 American Society of Anesthesiologists (ASA) physical status I-III adults aged 18 to 85 yr scheduled for elective non-cardiac surgery under general anesthesia at the Royal Columbian Hospital in New Westminster, BC, Canada. Exclusion criteria included surgical procedures scheduled for < 1 hour or > 6 hours, the need for intraoperative aortic cross-clamping, patients receiving spinal or epidural anesthesia only, known metabolic disorders (e.g., hypothyroidism), medications affecting core body temperature (e.g., l-thyroxine),18 pre-existing preoperative hypothermia (< 35.5°C) or hyperthermia (> 37.5°C), and the use of a transdermal medication patch.19
Randomization and intervention
A block randomization schedule was generated by SAS version 9.2 (SAS Institute, Cary, NC, USA) with random block lengths (4, 8, and 12) for each scheduled surgical duration stratum (< 2.5 hr or ≥ 2.5 hr) to ensure equal distribution of intervention by time. The statistician (D.J.M.), blinded to the randomization schedule, marked the sequences as either “Treatment A” or “Treatment B”. A 3M clinical data analyst encoded the sequences with “use of Bair Paws” and “control” and placed them in sequentially numbered, sealed, opaque envelopes, with separate sets for stratified surgical duration. The envelopes were kept in a locked cupboard in the anesthesia department office, which only research staff accessed. Encoded sequences were subsequently revealed to the statistician after data lock.
After hospital admission and obtaining informed consent, participants were assigned to either control or pre-warmed groups by opening the sequentially numbered, sealed, opaque envelopes based on scheduled duration of surgery. Nurses in the surgical preoperative unit were informed of group allocation immediately; the anesthesiologist and operating room nurses were informed before the patient was transferred to the operating room. Both groups received a warmed flannel blanket during admission as per routine institutional practice. The control group, in standard hospital gowns, received additional warmed flannel blankets on request in the preoperative period for at least 30 min, again as per institutional practice. The pre-warmed group received active pre-warming for at least 30 min via the disposable BairPaws full-body forced-air convective warming gowns in addition to requested warmed blankets. The BairPaws gown was worn in place of standard hospital gowns from hospital admission until discharge from the PACU. Lower body air inlet ports were connected to the BairPaws portable warming unit (3M BairPaws™, model 87500). Throughout the pre-warming period, study participants were allowed to adjust the temperature output of the device to best suit their own thermal comfort (i.e., without inducing perspiration). They were instructed to keep it as high as tolerated, required to maintain a minimum setting of 41°C with low fan output (97 W), and not permitted to switch the heating device off. The device’s maximum setting (high heat, high airflow) corresponds to an air temperature that stabilizes at 43 ± 3°C.
After surgical draping, intraoperative forced-air warming was instituted at the discretion of the anesthesiologist and continued until the end of surgery. Based on the required surgical exposure, either upper and/or lower body forced-air blankets (3M™ Bair Hugger™ Intraoperative Blankets, models 522 and 525) connected to a BairHugger warming unit (3M Canada, model 77500) were used in the control group, whereas in the pre-warming group, the BairPaws gown was folded, as per manufacturer instructions,20 to simulate an upper and/or lower body blanket.
The SpotOn™ temperature system (3M; St. Paul, MN, USA) was utilized throughout the perioperative period to measure core temperatures in both groups. This system consisted of a monitoring unit displaying the measured core temperature via a single-use disposable sensor placed on a patient’s forehead after randomization. The SpotOn provides a non-invasive measurement of core body temperature with a reported accuracy of ± 0.20°C between 31.0-37.0°C.21,22 Pre- and postoperative temperatures were manually recorded at specific time intervals including baseline (before the preoperative warming period), end of the pre-warming period (prior to leaving for the operating room), PACU arrival, and PACU discharge. Times of specific events were also recorded, including the induction of general anesthesia and the start of intraoperative forced-air warming. Intraoperative vital signs, including continuous core temperatures relayed from the SpotOn monitoring unit, were recorded using the S/5 Collect system (GE Healthcare Canada; Mississauga, ON, Canada). Institutional nursing protocols for temperature assessment were followed from hospital admission through to PACU discharge.
Total intraoperative intravenous crystalloid volumes (including those fluids administered at room temperature and those warmed to 37.0°C) were obtained from anesthetic and nursing records and ambient operating room temperatures were noted. Secondary outcomes of estimated blood loss, red blood cell transfusions, number of flannel blankets used, postoperative opioid consumption during the first 24 hr, and PACU and hospital LOS were also recorded. A standard morphine equivalent daily dose conversion was based on the 2015 Canadian Compendium of Pharmaceuticals and Specialties.18 Thermal comfort and anxiety were assessed using a self-report 0-10 rating scale (0 = very comfortable/no anxiety; 10 = very uncomfortable/anxious)23,24 prior to the induction of anesthesia and at discharge from the PACU. Pain was assessed using a self-reported numeric rating scale25,26 (0 = no pain; 10 = worst pain ever) 20 min after admission to the PACU and at PACU discharge. The occurrence of an SSI within 30 days postoperatively and at 90 days for surgical implants (e.g., hardware, screws, mesh) was determined by the participant’s surgeon and/or family doctor, according to National Surgical Quality Improvement Program criteria.27 Secondary outcome assessors were not blinded to the assigned treatment groups.
Statistical analysis
The primary outcome of intraoperative hypothermic magnitude was assessed by the AUC for core temperatures < 36°C between induction of anesthesia and leaving the operating room calculated using continuously recorded intraoperative core temperature data; the overall incidence of hypothermia (< 36°C) was also assessed. The duration of hypothermia relative to the procedure length (from time of induction of general anesthesia to the patient leaving the operating room) as a percentage of the case spent hypothermic (%CSH) was also calculated.
For the primary outcome of AUC for temperature < 36°C, no variability data were available to predict the sample size required to have 80% power to detect a median change of 2.0°C·hr−1. This is a meaningful difference that manifests as participants remaining 1°C below 36°C for at least two hours, an anticipated possibility based on the proportion of patients observed to be hypothermic even two hours post-induction of general anesthesia.16 We therefore powered the study to detect hypothermic exposure based on a decrease in the absolute incidence of intraoperative hypothermia for core temperature < 36°C, with interim analyses to determine sample size and power requirements for AUC analysis. A preliminary sample size estimate of 100 subjects per treatment group provided 80% power, with an alpha level of 0.05, to detect a relative decrease in the incidence of hypothermia by 38% (i.e., a decrease in the absolute incidence from 50% to 31%) and was based on previous work.16 After 100 participants had been enrolled, a blinded interim analysis was then conducted that confirmed sufficient power to detect differences in the AUC; the standard deviation of the AUC was 0.3°C·hr−1; thus, as few as four participants provide 80% power to detect the difference of 2.0°C·hr−1 identified as clinically significant. The sample size impact of analyzing by scheduled procedure length strata was not evaluated. No alterations to the study design were made, and the study continued until the full 200 participants were enrolled to detect changes in hypothermic incidence as intended.
Artefactual data from each participant’s core temperature data were eliminated in a systematic process whereby all values < 30°C were removed, after which a five-data point median filter was applied to the recorded temperatures (with each data point representing temperature data recorded every ten seconds). Changes of > 0.1°C per ten seconds were excluded on the basis of physiologic improbability.
The AUC and %CSH were assessed using the Wilcoxon rank-sum test. These data are reported as median [interquartile range (IQR)] and comparisons reported with median difference (MD). The Hodges-Lehmann estimator was used to calculate the 95% confidence interval (CI). The hypothermia incidence was assessed using a Chi-squared test and is reported with the odds ratio (OR) and 95% CI, calculated using logistic regression.
A Wilcoxon rank-sum test was also used for the secondary outcomes with MD and 95% CI calculated using the Hodges-Lehmann estimator. A Hochberg procedure, a simple sequential Bonferroni-type procedure,28 was used to adjust for multiple comparisons of secondary outcomes; with P < 0.05 for the three primary analyses, no adjustment was required by the Hochberg procedure.
Stratification by scheduled procedure duration was designed to ensure balanced procedure length between the two groups rather than to support subgroup analyses. Nevertheless, effect modification based on procedure duration was evaluated using an analysis of variance model including AUC, group, and scheduled duration strata, and the data for AUC, %CSH, and incidence of hypothermia are presented separately for procedures < 2.5 hr, procedures ≥ 2.5 hr, and overall.
The effects of actual procedure length on hypothermic incidence, %CSH, and AUC were estimated using regression of the raw data; similar analyses related to the influence of time to re-initiation of warming were conducted. Logistic regression was used to estimate the odds of increasing the incidence of hypothermia for each minute of delay between the end of pre-warming and the start of intraoperative warming. Furthermore, we conducted a post hoc regression analysis to evaluate the effect of the duration of pre-warming time on AUC for pre-warmed participants.
All available data were used and no imputation of missing data was performed. The statistician was blinded to treatment group until data lock and cleaning of artefactual data. The number needed to treat (NNT) to prevent additional cases of intraoperative hypothermia was calculated by taking the reciprocal of the difference in absolute risk of the incidence of hypothermia in pre-warmed vs control participants. The NNT to reduce the magnitude of hypothermia by 1°C·hr−1 was calculated by taking the reciprocal of the difference in the AUC for pre-warmed vs control participants.
The statistical software used for all analyses was SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
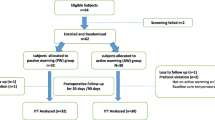
Of the 220 study subjects enrolled (Fig. 1), 20 were excluded after randomization: nine for surgical delay or cancellation, five for change to spinal anesthesia, and four because of information discovered before or just after induction of anesthesia that led to the participant being deemed ineligible (i.e., procedure involved aortic cross-clamping; ASA physical status IV; active SSI; hypothyroidism). In addition, two participants withdrew before the start of the study procedures. No participants were lost to follow-up. Data from 200 participants were analyzed. Baseline demographic data are listed in Table 1. Types of operations were general surgical (53.5%), orthopedic (16.0%), gynecologic (15.5%), and urologic procedures (12.0%).
At the end of pre-warming, 97 (96%) control and 94 (95%) pre-warmed participants met the minimum 30-min preoperative warming period. There was no difference between the pre-warmed vs control groups for the median [IQR] duration of preoperative warming or for the time delay between the end of pre-warming to the start of intraoperative warming (33 [26-51] min vs 37 [24-51] min, respectively; MD, 0 min; 95% CI, −5 to 5 min; P = 0.96) (Table 1). Pre-warmed participants were warmer than control participants at the end of the pre-warming period (36.9 [36.7-37.2] °C vs 36.8 [36.5-36.9] °C, respectively; MD, 0.2°C; 95% CI, 0.0 to 0.3°C; P = 0.004) (Table 2; Fig. 2). On PACU arrival, pre-warmed participants were also warmer than control participants (36.4 [36.0-36.8] °C vs 36.0 [35.6-36.4] °C, respectively; MD, 0.4°C; 95% CI, 0.2 to 0.4°C; P < 0.001).
The primary outcome of the median [IQR] AUC for the magnitude of intraoperative hypothermic exposure was significantly lower in the pre-warmed group compared with the control group (0.00 [0.00-0.12] °C·hr−1 vs 0.05 [0.00-0.36] °C·hr−1, respectively; MD, −0.01°C·hr−1; 95% CI, −0.04 to 0.00°C; P = 0.005) (Table 2; Fig. 3). When stratified by procedure duration, the difference in AUC between the pre-warmed and control groups remained for procedures ≥ 2.5 hr (0.01 [0.00-0.16] °C·hr−1 vs 0.11 [0.00-0.83] °C·hr−1, respectively; MD, −0.06°C·hr−1; 95% CI, −0.21 to −0.02°C·hr−1; P = 0.002), but not for procedures < 2.5 hr (0.00 [0.00-0.08] °C·hr−1 vs 0.00 [0.00-0.10] °C·hr−1, respectively; MD, 0.00°C·hr−1; 95% CI, 0.00 to 0.00°C·hr−1; P = 0.34). Interaction between the group and scheduled procedure duration strata was not significant (P = 0.06). Length of surgery did not alter the AUC for pre-warmed participants (P = 0.39), but did for control participants (P = 0.007), where every additional hour of actual procedure duration increased the AUC by 0.15°C·hr−1 (95% CI, 0.07 to 0.23°C·hr−1; P = 0.007) (Fig. 4).
At baseline, on enrolment into the study, 12/100 (12%) control and 11/99 (11%) pre-warmed participants were determined to be hypothermic. The incidence of intraoperative hypothermia (core temperature < 36°C) was lower in pre-warmed (n = 46) than control (n = 64) participants (47% vs 63%; OR, 0.50; 95% CI, 0.29 to 0.88; P = 0.02). At 60 min post-induction, the median [IQR] temperature in the pre-warmed group was higher than in the control group (36.3 [36.0-36.7] °C vs 36.0 [35.6-36.3] °C; MD, 0.3°C; 95% CI, 0.14 to 0.48°C; P < 0.001). Higher median [IQR] temperatures in pre-warmed vs. control participants were observed for more than three hours (Fig. 2) and at 180 min post-induction were 36.6 [36.2-36.9] °C vs 36.3 [35.8-36.6] °C (MD, 0.3°C; 95% CI, 0.0 to 0.6°C; P = 0.046).
There was a difference in the median [IQR] %CSH between pre-warmed and control participants (0.0 [0.0-26.8] % vs 13.6 [0.0-65.1] %, respectively; MD, −2.5%; 95% CI, −10.0 to 0.0%; P = 0.004) (Table 2; Fig. 3). When stratified by procedure duration, the difference in %CSH between pre-warmed and control participants remained with procedures ≥ 2.5 hr (2.3 [0.0-26.9] % vs 24.2 [6.6-76.4] %, respectively; MD, −9.9 %; 95% CI, 27.7 to -3.4%; P = 0.003), but not for procedures < 2.5 hr (0.0 [0.0-25.0] % vs 0.0 [0.0-59.0] %, respectively; MD, 0.0%; 95% CI, −2.2 to 0.0%; P = 0.20) (Fig. 4). No differences were noted in patient anxiety, thermal comfort, SSIs, estimated blood loss, red blood cell transfusions, postoperative opioid requirements, PACU LOS, or hospital LOS once adjusted for multiple comparisons (Table 3). Pre-warmed participants used fewer flannel blankets than control patients (2 [2-3] vs 4 [2-6], respectively; MD, −1; 95% CI, −2 to −1; P < 0.001).
Duration of pre-warming time had no effect on the AUC (P = 0.45). The delay between the end of the pre-warming period and initiation of intraoperative warming did not change the AUC for control participants (P = 0.39), but did so for pre-warmed participants (P < 0.001), where every minute of delay increased the AUC by 0.006°C·hr−1 (95% CI, 0.004 to 0.001; P < 0.001). The odds of hypothermia increased by 4.9% for every minute of delay encountered by pre-warmed participants between the end of the pre-warming period to initiation of intraoperative forced-air warming [OR, 1.05; 95% CI, 1.02 to 1.08].
Post hoc analysis of the reductions in incidence of hypothermia and AUC observed in our study showed an NNT of 5.9 (95% CI, 3.3 to 30.2) patients for pre-warming to prevent an additional case of perioperative hypothermia.
Discussion
In this study, a minimum duration of 30 min preoperative forced-air warming resulted in a decreased intraoperative magnitude of hypothermic exposure as well as incidence and duration of time spent hypothermic. Nevertheless, no clinically important differences in secondary outcomes were noted. The observed difference in magnitude of hypothermic exposure (−0.01°C·hr−1; 95% CI, −0.04 to 0.00°C·hr−1) was much smaller than the −2.0°C·hr−1 identified as a meaningful clinical difference in our sample size calculation.
We confirmed that increases in preoperative core temperatures are possible by increasing the heat content in the peripheral compartment with active pre-warming.14,29 While a duration of 30 min full-body forced-air warming for heat transference is considered sufficient,30,31 the most commonly studied target duration for pre-warming is 60 min.16 We confirmed that a minimum 30-min preoperative full-body forced-air warming, compared with warmed flannel blankets on demand, and combined with intraoperative warming, can reduce intraoperative hypothermic incidence by 16% (P = 0.02).
Intraoperative hypothermic exposure
Continuous perioperative temperature monitoring contrasts single point-in-time temperature measures (e.g., temperature on arrival to PACU) for defining the effectiveness of pre-warming in the perioperative period and provides new insights into its impact on intraoperative core temperature patterns. While the incidence of perioperative hypothermia is an important metric in evaluating associated adverse surgical outcomes, it should not be the sole measure.32 It is likely that hypothermic complications occur not only because of instantaneous declines in tissue temperatures to < 36°C (e.g., postoperative thermal discomfort), but also as a cumulative effect accrued throughout the surgical period (e.g., coagulopathy from altered enzymatic function). In this study, pre-warmed participants experienced a decreased magnitude of hypothermia and spent 13.7% less case time hypothermic (P = 0.004). Though few studies examine the association of integrated core temperatures with adverse perioperative outcomes, Sun et al. showed an association of increased erythrocyte transfusions with progressive increases of 1-8°C·hr−1. When the AUC < 37°C was 16.0°C·hr−1, the risk of transfusion requirements doubled.10 This is 80 times the difference observed in our study, which is likely due to the temperature reference of 37.0°C used by Sun et al. vs 36.0°C in this study.
Secondary outcomes analysis
No differences were noted between groups for the secondary outcomes of anxiety, thermal comfort, SSIs, estimated blood loss, red blood cell transfusions, opioid consumption, PACU LOS, or hospital LOS. This contrasts with previous studies where inadvertent hypothermia was associated with increased infectious and coagulopathic complications and improved satisfaction on arrival and discharge from PACU, surmised to result from the positive psychologic effects of a patient-controlled feature.2,4,23 This difference may be due to the use of a different evaluation tool or that analyses of relative changes in pre- to postoperative anxiety were not compared. Pre-warmed patients used fewer flannel blankets throughout the perioperative period, an expected finding as continuous heat provision for a forced-air warmer mitigates the need for more thermal insulation. Our analyses of secondary outcomes were limited by insufficient power to detect reported benefits related to the prevention of intraoperative hypothermia.
Factors impacting the efficacy of pre-warming
Meta-analysis of pre-warming studies indicates that it is effective in reducing redistribution hypothermia one hour after induction of general anesthesia by 0.42°C (95% CI, 0.27 to 0.57; P < 0.001).15 We noted a similar effect in our study with the mean temperature for the pre-warmed group 0.32°C higher than for the control group at 60 min after induction of general anesthesia (95% CI, 0.16 to 0.49°C; P < 0.001). Changes in clinical routine, differences in warming system efficacy, and attention to maintaining perioperative normothermia may contribute to the diminished redistribution hypothermia observed.33 In our study, we identified a further factor—delay to initiating intraoperative forced-air warming. For each minute of delay, the likelihood of a core temperature < 36°C increased by 4.9% (P < 0.001). Furthermore, each minute of delay increased the magnitude of hypothermic exposure by 0.006°C·hr−1 (P < 0.001) such that for an average 40-min delay, this results in 0.24 C·hr−1 more hypothermia. Potential benefits of preoperative forced-air warming are transient; heat stored in the peripheral compartment is lost to the cold environment once active pre-warming is stopped.
We observed no difference in AUC with longer warming prior to leaving for the operating room. Hence, increased duration of pre-warming beyond the 30 min as performed in this study may not result in better preservation of normothermia because of maintained thermal regulatory responses prior to induction of general anesthesia.
Participants in this study had a mean wait time of 40 min between the end of pre-warming and the start of intraoperative warming. Contributing factors include the institutional practice of initiating intraoperative warming only after surgical skin preparation and draping, variability in initiation time by attending anesthesiologists, and the time required for epidural catheter placement; general surgery operations typically used combined neuraxial-general anesthetic techniques. Reducing this delay should minimize the heat loss following pre-warming. Potential strategies include continuous forced-air warming via the BairPaws gown during transport and epidural insertion and detailing intraoperative warming in the surgical safety checklist.
Impact of pre-warming for clinical practice
Meaningful a priori-defined clinical differences in outcomes were not observed. This is due in part to the heterogeneity of patients and surgical procedures and the impact of delays on pre-warming effects. Furthermore, the National Institute for Care and Health Excellence standard of clinical significance, defined as a core temperature difference of 0.5°C for temperatures above 36.0°C, was not achieved in our study.34 Our observed core temperature difference of 0.4°C on arrival in the PACU may however be relevant in shorter and/or ambulatory operations because of the limited opportunity for intraoperative warming. This could impact LOS in PACU as previous studies have indicated that hypothermia delays appropriate PACU discharge based on medical and physiologic criteria alone.35 Increasing differences in hypothermic exposure by AUC, incidence, and %CSH between pre-warmed vs control participants in longer scheduled procedures likely indicate a larger surgical procedure with greater differences in metabolic heat production and heat loss.36 Pre-warming may therefore mitigate the additional heat loss by acting as a buffer until intraoperative forced-air warming and metabolic heat production restore normothermia.
Despite observations suggesting that the beneficial effect of pre-warming is limited to longer procedures, this study was not powered sufficiently for a subgroup analysis based on procedure duration and, as the interaction between treatment group and procedure duration strata was not significant, we are unable to draw firm conclusions about the utility of pre-warming for cases of shorter duration.
Limitations and future research
There were several additional limitations to our study. This trial was restricted to a single centre, which may not be generalizable to other centres with different ambient room temperatures, forced-air warming devices, and variation in application of upper/lower intraoperative forced-air warming blankets. The SpotOn sensor utilized for continuous perioperative temperature monitoring is a newer device with which practitioners may be unfamiliar. Comparable correlation, adequacy, and precision with respect to nasopharyngeal and sublingual thermometry have been reported.37 SpotOn has not been widely adopted in clinical practice and any potential limitations remain uncertain.
Future studies examining preoperative warming methods should have sufficient sample sizes to show clinically significant differences, including rates of SSIs and cardiac and transfusion events based on net benefit analysis.34 Limiting patient and surgical heterogeneity may reveal useful outcomes, focusing efforts to prevent inadvertent hypothermia in high-risk population groups like major vascular procedures in which surgical site infections carry significant patient morbidity and hospital costs.27 While cost analysis was not performed in our study, as this varies widely based on purchasing organization, our post hoc NNT analysis, indicating that 5.9 patients were required to prevent an additional case of hypothermia, may assist clinicians in evaluating the cost effectiveness of pre-warming interventions in preventing perioperative hypothermic complications. While criteria for significant differences in intraoperative core temperature are lacking, examining core temperature patterns throughout the perioperative period may help discriminate intervention efficacy in preventing adverse events.
Conclusion
A minimum 30-min period of preoperative forced-air warming can be practically implemented to effectively decrease intraoperative hypothermic exposure. While redistribution hypothermia still occurs despite pre- and intraoperative forced-air convective warming, their combined application results in a greater preservation of intraoperative normothermia compared with intraoperative forced-air warming alone. Minimizing delay from end of pre-warming to initiation of intraoperative warming may reduce the risk of hypothermic exposure. Further work is required to relate these benefits to significant differences in clinical outcomes.
References
Mahoney CB, Odom J. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA J 1999; 67: 155-63.
Billeter AT, Hohmann SF, Druen D, Cannon R, Polk HC Jr. Unintentional perioperative hypothermia is associated with severe complications and high mortality in elective operations. Surgery 2014; 156: 1245-52.
Kim YS, Jeon YS, Lee JA, et al. Intra-operative warming with a forced-air warmer in preventing hypothermia after tourniquet deflation in elderly patients. J Int Med Res 2009; 37: 1457-64.
Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008; 108: 71-7.
Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277: 1127-34.
Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology 1997; 87: 1318-23.
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med 1996; 334: 1209-15.
Hynson JM, Sessler DI, Moayeri A, McGuire J, Schroeder M. The effects of preinduction warming on temperature and blood pressure during propofol/nitrous oxide anesthesia. Anesthesiology 1993; 79: 219-28 discussion 21A-2A.
Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology 2008; 109: 318-38.
Sun Z, Honar H, Sessler DI, et al. Intraoperative core temperature patterns, transfusion requirement, and hospital duration in patients warmed with forced air. Anesthesiology 2015; 122: 276-85.
Hynson JM, Sessler DI. Intraoperative warming therapies: a comparison of three devices. J Clin Anesth 1992; 4: 194-9.
Kurz A, Kurz M, Poeschl G, Faryniak B, Redl G, Hackl W. Forced-air warming maintains intraoperative normothermia better than circulating-water mattresses. Anesth Analg 1993; 77: 89-95.
Wong PF, Kumar S, Bohra A, Whetter D, Leaper DJ. Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. Br J Surg 2007; 94: 421-6.
Just B, Trévien V, Delva E, Lienhart A. Prevention of intraoperative hypothermia by preoperative skin-surface warming. Anesthesiology 1993; 79: 214-8.
Akhtar Z, Hesler BD, Fiffick AN, et al. A randomized trial of prewarming on patient satisfaction and thermal comfort in outpatient surgery. J Clin Anesth 2016; 33: 376-85.
Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth 2008; 101: 627-31.
Hooven K. Preprocedure warming maintains normothermia throughout the perioperative period: a quality improvement project. J Perianesth Nurs 2011; 26: 9-14.
Canadian Pharmacists Association. Compendium of Pharmaceuticals and Specialities. 2015.
3M™ Canada Company. 3M Bair Hugger™ Model 750 Warming Unit Service Manual-2013. URL: http://multimedia.3m.com/mws/media/798433O/model-750-service-manual-english.pdf (accessed April 2018).
3M™ Canada Company. Comfort and Clinical Warming throughout the surgical journey-2012. URL: http://solutions.3msverige.se/3MContentRetrievalAPI/BlobServlet?lmd=1351595993000&locale=sv_SE&assetType=MMM_Image&assetId=1319240889745&blobAttribute=ImageFile (accessed April 2018).
Eshraghi Y, Nasr V, Parra-Sanchez I, et al. An evaluation of a zero-heat-flux cutaneous thermometer in cardiac surgical patients. Anesth Analg 2014; 119: 543-9.
Mäkinen MT, Pesonen A, Jousela I, et al. Novel zero-heat-flux deep body temperature measurement in lower extremity vascular and cardiac surgery. J Cardiothorac Vasc Anesth 2016; 30: 973-8.
Wagner D, Byrne M, Kolcaba K. Effects of comfort warming on preoperative patients. AORN J 2006; 84: 427-48.
Benotsch EG, Lutgendorf SK, Watson D, Fick LJ, Lang EV. Rapid anxiety assessment in medical patients: evidence for the validity of verbal anxiety ratings. Ann Behav Med 2000; 22: 199-203.
Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain 2011; 152: 2399-404.
Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011; 41: 1073-93.
Compoginis JM, Katz SG. American College of Surgeons National Surgical Quality Improvement Program as a quality improvement tool: a single institution’s experience with vascular surgical site infections. Am Surg 2013; 79: 274-8.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995; 57: 289-300.
Camus Y, Delva E, Sessler DI, Lienhart A. Pre-induction skin-surface warming minimizes intraoperative core hypothermia. J Clin Anesth 1995; 7: 384-8.
Connelly L, Cramer E, DeMott Q, et al. The optimal time and method for surgical prewarming: a comprehensive review of the literature. J Perianesth Nurs 2017; 32: 199-209.
Sessler DI, Schroeder M, Merrifield B, Matsukawa T, Cheng C. Optimal duration and temperature of prewarming. Anesthesiology 1995; 82: 674-81.
Hopf HW. Perioperative temperature management: time for a new standard of care? Anesthesiology 2015; 122: 229-30.
Madrid E, Urrútia G, Roqué i Figuls M, et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev 2016; 4: CD009016.
National Institute for Care and Health Excellence. Hypothermia: prevention and Management in Adults Having Surgery-Clinical guideline [CG65]; 2008. URL: https://www.nice.org.uk/guidance/cg65 (accessed April 2018).
Kiekkas P, Poulopoulou M, Papahatzi A, Souleles P. Effects of hypothermia and shivering on standard PACU monitoring of patients. AANA J 2005; 73: 47-53.
Roe CF. Effect of bowel exposure on body temperature during surgical operations. Am J Surg 1971; 122: 13-5.
Iden T, Horn EP, Bein B, Böhm R, Beese J, Höcker J. Intraoperative temperature monitoring with zero heat flux technology (3M SpotOn sensor) in comparison with sublingual and nasopharyngeal temperature: an observational study. Eur J Anaesthesiol 2015; 32: 387-91.
Acknowledgements
The authors thank all nurses and physicians in the Pre-Admission Clinic, Surgical Day Care Unit, Operative Room, and Post Anesthetic Care Unit at Royal Columbian Hospital/Fraser Health Authority as well as the Pediatric Anesthesia Research Team for their support of this study
Conflict of interest
Dan Morse is a salaried employee of 3M.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Aaron Lau contributed to study planning, recruited subjects, collected data, data interpretation, and wrote and edited the manuscript. Nasim Lowlaavar contributed to study planning, recruited subjects, collected data, provided data interpretation, maintained study databases, and wrote and edited the manuscript. Aaron Lau and Nasim Lowlaavar contributed equally to this manuscript. Erin M. Cooke contributed to study planning, completed the ethics application, recruited subjects, collected data, maintained study databases, and critically reviewed the manuscript. Nicholas West contributed to study planning, recruited subjects, provided data interpretation, and critically reviewed the manuscript. Alexandra German contributed to patient recruitment and data collection and critically reviewed the manuscript. Dan J. Morse was the sole contributor to the statistical analysis of collected data and critically reviewed the manuscript. Matthias Görges contributed to the study design, data analysis and interpretation, and generation of figures and critically reviewed the manuscript. Richard N. Merchant contributed to the conception and design of the study and data interpretation and critically reviewed the manuscript.
Financial support and sponsorship
3M Canada funded this trial and provided the 3M BairPaws™ system (portable warming unit, temperature controller, and disposable BairPaws™ warming gowns) and the 3M SpotOn temperature system (portable temperature display unit and sensors).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lau, A., Lowlaavar, N., Cooke, E.M. et al. Effect of preoperative warming on intraoperative hypothermia: a randomized-controlled trial. Can J Anesth/J Can Anesth 65, 1029–1040 (2018). https://doi.org/10.1007/s12630-018-1161-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-018-1161-8