Abstract
Background
As the use of ultrasound for regional anesthesia has increased, many studies have examined the distribution of local anesthetic and the location of the needle tip. Nevertheless, the relationship between motor stimulation threshold and distribution of local anesthetic is unclear. The aim of this study was to compare block onset time, distribution of local anesthetic, and location of the needle tip at two different motor stimulation thresholds, i.e., 0.2 and 0.5 mA, used in combination with ultrasound guidance.
Methods
This study included 94 patients undergoing arthroscopic shoulder surgery with ultrasound-guided interscalene brachial plexus block (ISBPB) plus nerve stimulation. Patients were randomized into two groups for the ISBPB procedure, i.e., when an evoked motor response was obtained at a current intensity of either 0.2 mA (Group 0.2) or 0.5 mA (Group 0.5). Block onset time, location of the needle tip, and distribution of local anesthetic were assessed.
Results
A response was elicited at the appropriate motor stimulation threshold in 88 patients (Group 0.2 = 43; Group 0.5 = 45). Block failure occurred in only three patients, all of whom were in Group 0.5. The mean [standard deviation (SD)] of block onset time was 8.0 (4.1) min in Group 0.2 and 11.4 (5.9) min in Group 0.5 [mean difference, 3.4 min; 95% confidence interval (CI), 1.2 to 5.9; P = 0.003]. The needle tip was located at a intraplexus position in 33 (77%) patients in Group 0.2 and in 15 (33%) patients in Group 0.5 (difference in proportion, 43%; 95% CI, 23 to 59; P < 0.001). The intramuscular spreading of local anesthetic occurred in 0 (0%) patients in Group 0.2 and in 8 (18%) patients in Group 0.5 (difference in proportion, 18%; 95% CI, 6 to 31; P = 0.007).
Conclusion
The onset time of the block was significantly faster with a motor stimulation threshold of 0.2 mA than with a threshold of 0.5 mA.
Résumé
Contexte
Avec l’Augmentation de l’utilisation de l’échographie en anesthésie régionale, de nombreuses études se sont penchées sur la distribution de l’anesthésique local et à l’emplacement de la pointe de l’aiguille. Le rapport entre le seuil de stimulation motrice et la répartition de l’anesthésique local reste néanmoins peu clair. Le but de cette étude a été de comparer le délai d’apparition du bloc, la distribution de l’anesthésique local et l’emplacement de la pointe de l’aiguille pour deux seuils différents de stimulation motrice, c’est-à-dire, 0,2 et 0,5 mA, utilisés en association avec le guidage échographique.
Méthodes
Cette étude a inclus 94 patients subissant une chirurgie arthroscopique de l’épaule avec bloc interscalénique échoguidé du plexus brachial (ISBPB) plus stimulation nerveuse. Les patients ont été randomisés dans deux groupes pour la procédure d’ISBPB, en fonction des potentiels évoqués en réponse à une intensité de courant de 0,2 mA (Groupe 0,2) ou 0,5 mA (Groupe 0,5). Le délai d’installation du bloc, l’emplacement de la pointe de l’aiguille et la distribution de l’anesthésique local ont été évalués.
Résultats
Une réponse au seuil de stimulation motrice appropriée a été obtenue chez 88 patients (Groupe 0,2 = 43; Groupe 0,5 = 45). Un échec du bloc n’a été constaté que chez trois patients qui étaient tous dans le Groupe 0,5. Le délai moyen (écart-type [ÉT]) d’installation du bloc a été de 8,0 (4,1) minutes dans le Groupe 0,2 et 11,4 (5,9) minutes dans le Groupe 0,5 [différence moyenne, 3,4 minutes; intervalle de confiance à 95 % (IC) 1,2 à 5,9; P = 0,003]. La pointe de l’aiguille était en position intraplexique chez 33 (77 %) patients du Groupe 0,2 et chez 15 (33 %) patients du Groupe 0,5 (différence en pourcentage, 43 %; IC à 95 %, 23 à 59; P = 0,001). Une propagation intramusculaire de l’anesthésique local a eu lieu chez 0 (0%) patient dans le Groupe 0,2 et chez 8 (18%) patients dans le Groupe 0,5 (différence en pourcentage, 18 %; IC à 95 %, 6 à 31; P = 0,007).
Conclusion
Le délai d’installation du bloc a été significativement plus court avec un seuil de stimulation motrice de 0,2 mA qu’avec un seuil de 0,5 mA.
Similar content being viewed by others
Interscalene brachial plexus block (ISBPB) involves blockade of the proximal roots of the brachial plexus at C4-C7 to provide anesthesia and analgesia for shoulder surgery.1 Interscalene brachial plexus block can be performed using ultrasound guidance alone or in combination with conventional methods, such as patient-reported paresthesias or nerve stimulator techniques.
Ultrasound-guided regional anesthesia has the advantages of decreasing the frequency of needle redirection and allowing the clinician to visualize the needle and nerve in real time while performing the block. As a result, there has been an increase in the use of ultrasound-guided ISBPB.2,3 Nevertheless, ISBPB with nerve stimulation is still commonly performed, with or without ultrasound guidance. When ISBPB is performed by experienced anesthesiologists, evidence suggests that ultrasound-guided ISBPB with nerve stimulation and ISBPB using nerve stimulation alone have similar efficacy.4
As the use of ultrasound for regional anesthesia has increased, much research has been conducted regarding the distribution of local anesthetic and the location of the needle tip.5,6 When ISBPB is performed using nerve stimulation, injection of local anesthetic is performed at the location eliciting an evoked motor response at a current intensity of 0.2-0.5 mA.7,8 Although the use of peripheral nerve electrical stimulation is still commonly used in clinical practice, the relationship between motor stimulation threshold and distribution of local anesthetic is unclear. Therefore, this study was conducted to evaluate block onset time, distribution of local anesthetic, and location of the needle tip using ultrasound-guided ISBPB with nerve stimulation when an evoked motor response was obtained at a current intensity of either 0.2 mA or 0.5 mA.
Methods
The study was approved by our institutional ethics committee (Hanyang University Hospital Institutional Review Board, HYUH 2012-05-006-001) and informed consent was obtained from all patients before registration in the study. Ninety-four patients (American Society of Anesthesiologists physical status I-III, aged 18-75 yr) scheduled for arthroscopic shoulder surgery under ultrasound-guided ISBPB with nerve stimulation were recruited from July 2012 to April 2013. Exclusion criteria included age < 18 yr, significant coagulopathy, infection at the injection site, allergy to local anesthetics, severe cardiopulmonary disease, known neuropathy, diabetes, and refusal to participate in the study.
The patients were randomly assigned to one of two groups for the ISBPB procedure, i.e., when an evoked motor response was elicited at a current intensity of either 0.2 mA (Group 0.2) or 0.5 mA (Group 0.5). Randomization was performed using a computer-generated randomization table developed by the principal investigator.
All ultrasound-guided ISBPBs were performed by a single anesthesiologist with experience in ultrasound-guided nerve blocks and an assistant. An observer assessed the ultrasound image until the end of the injection of local anesthetic. The patients, anesthesiologist, and observer were blinded to the stimulation current used. Only the assistant manipulating the nerve stimulator was aware of the group assignment to either Group 0.2 or Group 0.5. Intramuscular midazolam 0.05 mg·kg−1 was administered to the patients for anxiolysis before their arrival in the operating room, and peripheral intravenous access was established. After entering the operating room, standard monitoring was initiated, including noninvasive blood pressure monitoring, electrocardiography, and pulse oximetry. Patients were placed in the supine position with their head turned 45° away from the operative shoulder. The interscalene area was prepared with antiseptic solution (chlorohexidine gluconate), and a high frequency (6-12 MHz) linear probe of a MicroMaxx® ultrasound system (SonoSite Inc., Bothwell, WA, USA) was placed over the brachial plexus in the interscalene groove (at the level of the roots and trunks). The ultrasound probe was covered with a sterile sheet. After skin infiltration with 1% lidocaine 1 mL, a 50-mm 22G insulated Tuohy needle (PAJUNK®, Geisingen, Germany) was advanced toward the region between the two most superficial hypoechogenic circles (representing the C5 and C6 nerve roots) using the in-plane technique. Using the Stimuplex® HNS11 nerve stimulator (B. Braun, Melsungen, Germany), the stimulation frequency and pulse duration were set at 2 Hz and 0.1 msec, respectively. The current intensity was initially set to deliver 1 mA at 1 cm before reaching the lateral border of the two most superficial hypoechogenic circles. The needle tip was advanced until an evoked motor response appeared. Once a response was observed, the current intensity was lowered to 0.2 mA (Group 0.2) or 0.5 mA (Group 0.5). The needle tip was advanced until an evoked motor response reappeared, at which time 0.5% ropivacaine 20 mL was injected. Aspiration was performed frequently during this injection. If no evoked motor response was obtained, it was assumed that the needle tip had passed through the anterior border of the brachial plexus, and the patient was excluded from the study.
During the procedure, the practicing anesthesiologist controlled the factors that could potentially influence the needle placement, including approach, determination of the skin entry point, control of needle direction, and needle advancement. To control these factors, the anesthesiologist first used the posterior approach described by van Geffen et al. to position the needle tip between the two most superficial large hypoechogenic circles.9 Second, the anesthesiologist determined the skin entry point as the point on the skin where an imaginary straight line bisects the space between the hypoechogenic circles. Finally, the anesthesiologist tried to move the needle parallel to the imaginary bisecting line.
Assessments of the degree of sensory and motor block were performed every five minutes for 30 min after injecting the local anesthetic. Assessment of the degree of sensory blockade was performed using a pinprick test in the C5, C6, and C7 dermatomes: 0 = normal sensation within the nerve distribution (no block); 1 = blunted sensation within the nerve distribution (hypoalgesia); and 2 = absence of sensation within the nerve distribution (anesthesia). Assessment of the degree of motor blockade was performed by determining the extent of shoulder abduction (deltoid) and elbow flexion (biceps): 1 = full motor function; 2 = limited motion; 3 = almost inability; and 4 = total inability. Successful nerve block was defined as complete sensory (score = 2) and motor (score = 4) blockade within 30 min of performing the ISBPB. The onset time of block was defined as the time from injection of local anesthetic to successful nerve block. Surgery was performed via arthroscopy with the patient in the beach chair position and receiving moderate sedation with propofol and supplemental oxygen by a venturi mask. If the block failed, general anesthesia was administered.
We evaluated the procedure time (from placing an ultrasound probe in the interscalene area to injection of local anesthetic), onset time of block, and postoperative pain in the postanesthesia care unit (determined by a visual analogue scale score in which 0 = no pain and 10 = worst pain imaginable). During the performance of the block, the observer investigated the needle depth, the relationship between the nerves and needle tip, and the distribution pattern of local anesthetic. The relationship between the nerves and needle tip was designated as intraplexus or periplexus. Intraplexus was defined as the location of the needle tip in the brachial plexus sheath between the two most superficial hypoechogenic circles, and periplexus was defined as the location of the needle tip outside the brachial plexus sheath (Fig. 1).5 The success rate and block onset time were compared between the two groups. The distribution of local anesthetic was designated as circumferential, non-circumferential, or intramuscular spreading. Circumferential spreading was defined as the local anesthetic completely enveloping the nerve roots (i.e., C5, C6, and C7) in the interscalene groove. Non-circumferential spreading was defined as local anesthetic incompletely enveloping the nerve roots in the groove, and intramuscular spreading was defined as local anesthetic spreading in the middle scalene muscle (Fig. 2). Postoperative neurologic symptoms were assessed at 24 and 48 hr after surgery.
Pattern of local anesthetic distribution. A) Circumferential spreading. B) Non-circumferential spreading. C) Intramuscular spreading. The C5, C6, and C7 nerve roots of the brachial plexus are identified. The asterisks delineate the extent of local anesthetic spreading. ASM = anterior scalene muscle; MSM = middle scalene muscle
The primary outcome was the onset time of block. An ultrasound-guided injection deep to the fascial sheath at the supraclavicular fossa or popliteal fossa results in very rapid onset time of blockade. It would seem intuitive that a lower stimulation threshold will likely be associated with intraplexus positioning. Therefore, we determined the onset time of block as the primary outcome. Secondary outcomes included the needle depth, procedure time, relationship between the nerves and needle tip, and distribution pattern of local anesthetic.
Statistical analysis
In a pilot study of 20 patients, the onset time of block was seven minutes (Group 0.2) and 12 min (Group 0.5). A sample size of 47 patients in each group was estimated for the two-sided test of differences in onset time of block to achieve a type I error (α) of 0.05, and a power (1 − β) of 90% to detect a difference of five minutes. Based on data from a pilot study, we assumed a standard deviation (SD) of seven minutes.
The statistical analyses were performed using PASW Statistics 18.0 for Windows (SPSS® Inc., Chicago, IL, USA). Continuous variables are presented as mean (SD) and categorical variables are presented as number (%). Chi square tests or Fisher’s exact tests were used for categorical variables, and the Mann-Whitney U test was used for continuous variables. The Bonferroni method was used for multiple variable comparisons. All reported P values are two sided.
Results
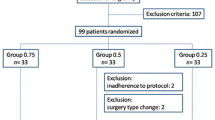
Ninety-four patients were recruited, but six were excluded because no evoked motor response was elicited during performance of the block (four patients in Group 0.2 and two in Group 0.5).Therefore, 88 patients completed the study (Fig. 3). The demographic data and success ratios are presented in Table 1. Block failure occurred in only three patients, all of whom were in Group 0.5. Nevertheless, the success ratio was not different between the two groups (P < 0.242). The duration of surgery, procedure time, and postoperative pain scores were similar between the two groups (excluding the three patients with an unsuccessful nerve block). The mean (SD) block onset time was 8.0 (4.1) min in Group 0.2 and 11.4 (5.9) min in Group 0.5 (mean difference, 3.4 min; 95% confidence interval [CI], 1.2 to 5.9; P = 0.003) (Table 2). The needle tip was located at the intraplexus in 33 of 43 (77%) patients in Group 0.2 and in 15 of 45 (33%) patients in Group 0.5 (difference in proportion, 43%; 95% CI, 23 to 59; P < 0.001) (Fig. 4A). There was intramuscular spreading of local anesthetic in 0 of 43 (0%) patients in Group 0.2 and in 8 of 45 (18%) patients in Group 0.5 (difference in proportion, 18%; 95% CI, 6 to 31; P = 0.007) (Fig. 4B). There was circumferential spreading of local anesthetic in 28 of 43 (65%) patients in Group 0.2 and in 19 of 45 (42%) patients in Group 0.5 (difference in proportion, 23%; 95% CI, 2 to 41; P = 0.034) (Fig. 4B). When the patients were divided into intraplexus and periplexus groups (48 and 37 patients, respectively), the success rate did not differ between two groups (P < 0.090). The block onset time was 3.8 min faster in the intraplexus group than in the periplexus group (P = 0.001). Complications, such as intravascular injection, vascular trauma, or seizures did not occur during any block procedures. Similarly, no neurologic symptoms were observed in any patients at 24 or 48 hr postoperatively.
A) Location of needle tip according to motor stimulation threshold. B) Distribution of local anesthetic according to motor stimulation threshold. The needle tip was located at the intraplexus in 77% of patients in Group 0.2 and 33% of patients in Group 0.5 (P < 0.001). The intramuscular spreading of local anesthetic was 0% in Group 0.2 and 18% in Group 0.5 (P = 0.007). The circumferential spreading of local anesthetic was 65% in Group 0.2 and 42% in Group 0.5 (P = 0.034)
Discussion
In this study, we found that the block onset time was faster in Group 0.2 than in Group 0.5 (mean difference, 3.4 min; 95% CI, 1.2 to 5.9). Furthermore, in Group 0.2, the needle tip was more often located at the intraplexus and the local anesthetic was more frequently distributed as circumferential spreading when compared with Group 0.5. These results suggest that the use of a motor stimulation threshold of 0.2 mA for ultrasound-guided ISBPB will provide rapid onset time and increased success rates. Nevertheless, the risk of nerve injury may be increased if the needle tip cannot be clearly visualized.
In a study of ultrasound-guided ISBPB, Spence et al.5 showed that the success rate and block onset time did not differ between intraplexus and periplexus injections. Although no neurologic complications were noticed, the authors suggested that periplexus injections would reduce the risk of such complications by decreasing the possibility of needle-to-nerve contact. Even so, in this study the block onset time was 3.8 min faster in the intraplexus group than the periplexus group, and the success rate did not differ between the two groups.
In a study of ultrasound-guided ISBPB with nerve stimulation, Lang et al.6 examined the clinical block characteristics for different local anesthetic distribution patterns. The results showed that the characteristics did not differ between the group in which local anesthetic completely enveloped the nerves in the interscalene groove and the group in which the nerves were only partly enveloped. The authors of a recent study sought to determine the maximal effective needle-to-nerve distance for ultrasound-guided ISBPB, and they concluded that placing the needle tip in the middle scalene muscle may be a reasonable goal to achieve a successful analgesic ISBPB.10 Nevertheless, in our study of the eight patients who exhibited intramuscular spreading, three patients had an unsuccessful block. Intramuscular spreading was noticed only in patients in Group 0.5 and in those with a periplexus needle tip location. Therefore, the faster block onset time in Group 0.2 was likely attributed to the intraplexus location of the needle tip and to the circumferential spreading of local anesthetic. Even so, it should be pointed out that a difference in onset time of approximately three minutes may have little clinical significance.
It was noted in previous reports that the effectiveness of ISBPB with nerve stimulation did not differ when performed alone or with ultrasound guidance by experienced anesthesiologists.4,11 Nevertheless, another study reported a higher success rate and a lower complication rate using ultrasound-guided ISBPB without nerve stimulation vs previous studies using ISBPB with nerve stimulation.12 In our study, the lower stimulation threshold was associated with more accurate placement of the needle tip and a faster block onset time. Therefore, we suggest that ultrasound-guided ISBPB with nerve stimulation is a useful technique, but the selection of stimulation threshold is an important consideration.
When an evoked motor response occurs at a low stimulation threshold, it is typically assumed that the needle tip is positioned close to the nerve. The possibility of intraneural needle tip placement has previously been reported if the stimulation threshold is < 0.5 mA,13,14 and adequate blocks have been reported at a stimulation threshold of 1.0 mA and a pulse duration of 0.1 msec.8,15 Nevertheless, we did not observe any intraneural needle tip placement in our study at a stimulation intensity of 0.2 mA. It is likely that the use of ultrasound to evaluate needle tip placement and the layers of the nerve is limited.14,16
Our study has some limitations. First, we cannot exclude the influence of ultrasound guidance on our results. Despite standardization of approach, needle insertion point, and needle advancement, the attending anesthesiologist could visualize the needle tip and its relationship to the plexus throughout the block. Direct observation of the needle tip may override the complementary information from nerve stimulation. Second, measuring block onset five minutes after the injection could potentially have masked any onset time occurring prior to five minutes. Finally, evaluating the spreading pattern of local anesthetic was difficult because both intramuscular and intra-sheath spreading of local anesthetic appeared in some patients. In these cases, we classified the distribution based on the area with the greater degree of local anesthetic spread.14,16
In conclusion, we found that the block onset time for ultrasound-guided ISBPB was significantly faster with a motor stimulation threshold of 0.2 mA than with a motor stimulation threshold of 0.5 mA. The faster onset time was generally associated with the intraplexus needle tip location and the circumferential spreading of local anesthetic.
References
Kapral S, Greher M, Huber G, et al. Ultrasonographic guidance improves the success rate of interscalene brachial plexus blockade. Reg Anesth Pain Med 2008; 33: 253-8.
Fredrickson MJ, Ball CM, Dalgleish AJ. A prospective randomized comparison of ultrasound guidance versus neurostimulation for interscalene catheter placement. Reg Anesth Pain Med 2009; 34: 590-4.
Orebaugh SL, Williams BA, Kentor ML. Ultrasound guidance with nerve stimulation reduces the time necessary for resident peripheral nerve blockade. Reg Anesth Pain Med 2007; 32: 448-54.
Salem MH, Winckelmann J, Geiger P, Mehrkens HH, Salem KH. Electrostimulation with or without ultrasound-guidance in interscalene brachial plexus block for shoulder surgery. J Anesth 2012; 26: 610-3.
Spence BC, Beach ML, Gallagher JD, Sites BD. Ultrasound-guided interscalene blocks: understanding where to inject the local anaesthetic. Anaesthesia 2011; 66: 509-14.
Lang RS, Kentor ML, Vallejo M, Bigeleisen P, Wisniewski SR, Orebaugh SL. The impact of local anesthetic distribution on block onset in ultrasound-guided interscalene block. Acta Anaesthesiol Scand 2012; 56: 1146-51.
Hadzic A, Vloka JD, Claudio RE, Hadzic N, Thys DM, Santos AC. Electrical nerve localization: effects of cutaneous electrode placement and duration of the stimulus on motor response. Anesthesiology 2004; 100: 1526-30.
Wiesmann T, Borntrager A, Vassiliou T, et al. Minimal current intensity to elicit an evoked motor response cannot discern between needle-nerve contact and intraneural needle insertion. Anesth Analg 2014; 118: 681-6.
van Geffen GJ, Rettig HC, Koornwinder T, Renes S, Gielen MJ. Ultrasound-guided training in the performance of brachial plexus block by the posterior approach: an observational study. Anaesthesia 2007; 62: 1024-8.
Albrecht E, Kirkham KR, Taffe P, et al. The maximum effective needle-to-nerve distance for ultrasound-guided interscalene block: an exploratory study. Reg Anesth Pain Med 2014; 39: 56-60.
Desmet M, Braems H, Reynvoet M, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth 2013; 111: 445-52.
Davis JJ, Swenson JD, Greis PE, Burks RT, Tashjian RZ. Interscalene block for postoperative analgesia using only ultrasound guidance: the outcome in 200 patients. J Clin Anesth 2009; 21: 272-7.
Steinfeldt T, Schwemmer U, Volk T, et al. Nerve localization for peripheral regional anesthesia. Recommendations of the German Society of Anaesthesiology and Intensive Care Medicine. Anaesthesist 2014; 63: 597-602.
Bigeleisen PE, Moayeri N, Groen GJ. Extraneural versus intraneural stimulation thresholds during ultrasound-guided supraclavicular block. Anesthesiology 2009; 110: 1235-43.
Vassiliou T, Muller HH, Ellert A, et al. High- versus low-stimulation current threshold for axillary plexus blocks: a prospective randomized triple-blinded noninferiority trial in 205 patients. Anesth Analg 2013; 116: 247-54.
Chin KJ, Perlas A, Chan VW, Brull R. Needle visualization in ultrasound-guided regional anesthesia: challenges and solutions. Reg Anesth Pain Med 2008; 33: 532-44.
Conflicts of interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was registered with the CRIS clinical trial registry (http://cris.nih.go.kr/cris/, KCT0000582).
L’étude a été enregistrée dans le registre des essais cliniques CRIS (http://cris.nih.go.kr/cris/, KCT0000582).
Rights and permissions
About this article
Cite this article
Jeong, J.S., Shim, J.C., Shim, J.H. et al. A comparison of motor stimulation threshold in ultrasound-guided interscalene brachial plexus block for arthroscopic shoulder surgery: a randomized trial. Can J Anesth/J Can Anesth 63, 461–467 (2016). https://doi.org/10.1007/s12630-015-0553-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-015-0553-2