Abstract
Purpose
Previous studies have found that most cerebral oximeters are subject to inaccuracies secondary to extracranial contamination of the cerebral oximetric signals. We hypothesized that the more advanced second-generation FORE-SIGHT ELITE cerebral oximeter would be significantly less affected by extracranial tissue hypoxemia than the more widely used first-generation INVOS™ 5100C monitor.
Methods
Twenty healthy volunteers aged 18-45 yr had the INVOS and FORE-SIGHT probes placed on their forehead in a random sequence while in the supine position. A pneumatic head cuff was then placed around each volunteer’s head just below both the oximeter and a concomitantly placed scalp forehead pulse oximeter probe. The subjects’ scalp cerebral oxygen saturation (SctO2) values were measured and compared using the two different devices in sequence, both before and after scalp tissue ischemia was induced by the pneumatic cuff.
Results
Extracranial ischemia resulted in a significant reduction in SctO2 values from baseline in both devices. The INVOS 5100C recorded a median [interquartile range] decrease in SctO2 from baseline at five minutes of 15.1% [12.6 - 17.6], while that recorded by the FORESIGHT ELITE device was 8.6% [4.0 -12.3] at five minutes (median difference, 7.9%; 99% confidence interval, 1.9 to 16.5; P = 0.002).
Conclusion
Updated technological algorithms employed in the FORE-SIGHT ELITE cerebral oximeter may be responsible for less extracranial contamination than was observed in the previous-generation INVOS 5100C device. The impact that this extracranial contamination may have on the clinical use of these devices remains to be determined.
Résumé
Objectif
Selon les études réalisées par le passé, la plupart des oxymètres cérébraux sont sujets à des inexactitudes suite à une contamination extracrânienne des signaux d’oxymétrie cérébrale. Nous avons émis l’hypothèse que l’oxymètre cérébral FORE-SIGHT ELITE, l’oxymètre cérébral de deuxième génération le plus innovant à ce jour, serait significativement moins affecté par l’hypoxémie des tissus extracrâniens que le moniteur de première génération INVOS™ 5100C, plus répandu.
Méthode
Les sondes des appareils INVOS et FORE-SIGHT ont été placées selon une séquence aléatoire sur le front de vingt volontaires sains âgés de 18 à 45 ans en position allongée sur le dos. On a ensuite placé un bandeau gonflable autour de la tête de chaque participant, juste au-dessous de l’oxymètre, et placé une sonde d’oxymètre de pouls simultanément sur le front. La saturation en oxygène cérébral sur le scalp (SctO2) des participants a été mesurée et comparée à l’aide des deux appareils en séquence, avant et après provocation d’une ischémie des tissus du scalp par le bandeau gonflable.
Résultats
L’ischémie extracrânienne a entraîné une réduction significative des valeurs de SctO2 comparativement aux valeurs de base obtenues avec les deux appareils. L’oxymètre INVOS 5100C a enregistré une réduction moyenne [écart interquartile] de la SctO2 à cinq minutes de 15,1 % [12,6 – 17,6], alors que le FORE-SIGHT ELITE a enregistré une réduction de 8,6 % [4,0 −12,3] à cinq minutes comparativement aux valeurs de base (différence médiane, 7,9 %; intervalle de confiance 99 %, 1,9 à 16,5; P = 0,002).
Conclusion
Les algorithmes technologiques plus récents employés par l’oxymètre cérébral FORE-SIGHT ELITE pourraient expliquer la contamination extracrânienne moindre observée avec cet appareil comparativement à celle observée avec le dispositif de première génération évalué ici, l’INVOS 5100C. L’impact de cette contamination extracrânienne sur l’utilisation clinique de ces dispositifs doit encore être déterminé.
Similar content being viewed by others
Patients undergoing cardiac surgery and various operations in the beach chair position may be at risk for cerebral ischemia and subsequent neurologic injury.1–3 Early detection of any cerebral ischemia may allow clinicians to institute therapies that prevent permanent injury to the central nervous system (CNS). Several technologies have been applied in the perioperative setting that may permit real-time detection of cerebral ischemia.3,4 Multichannel electroencephalographic (EEG) monitoring can measure the presence or absence of ischemic changes in the brain. Nevertheless, the use of multichannel EEG monitoring is resource intensive and usually requires the full-time attendance of a technician and/or neurologist to monitor and interpret the complex electrical activity of the brain on a continuous basis. Serial measurements of jugular venous bulb oxygen saturation (SjvO2) allow early detection of CNS ischemia, but the technology is highly invasive (i.e., requiring placement of catheters into the jugular bulb) and is now rarely used outside of research settings.4
In contrast, near-infrared spectroscopy (NIRS)-based cerebral oximetry is a noninvasive technology that is relatively easy to use and specifically developed to allow clinicians to detect and treat cerebral ischemia in the operating room.1–5 The degree of oxygen saturation in cerebral tissue (SctO2) can be determined through the use of multi-wavelength light emitters and detectors,.6 Derived SctO2 signals represent a mixture of venous and arterial blood (approximately 70% and 30%, respectively) in the cerebral frontal cortex.3–5 The INVOS™ 5100C oximeter (Medtronic, Minneapolis, MN, USA) uses two wavelengths of infrared light (730 and 810 nm), while the newer generation FORESIGHT-ELITE (CAS Medical Systems Inc, Branford, CT, USA) uses five wavelengths (680, 730, 770, 805, and 870 nm)Footnote 1 of light that may enhance accuracy and reduce extracranial contamination. As brain oxygen demand remains relatively stable under anesthesia, changes in SctO2 typically represent reductions in cerebral oxygen supply, which are often due to reductions in blood pressure, carbon dioxide partial pressure, cardiac output, hemoglobin concentration, and/or arterial oxygen content.5–12
The accuracy of SctO2 measurements under a variety of clinical conditions is essential if appropriate assessment and management of brain oxygenation is to be possible. Nevertheless, previous studies suggest that extracranial contamination can influence the measurement of SctO2 and result in measured values that do not entirely reflect the status of oxygenation in the brain.1,13
The aim of the present investigation was to compare the effect of extracranial contamination on SctO2 values as measured by the FORE-SIGHT ELITE vs the INVOS 5100C monitor. We hypothesized that the SctO2 values measured by the second-generation FORE-SIGHT ELITE would be significantly less affected by extracranial contamination from superficial tissue ischemia than those measured by the first-generation INVOS 5100C monitor.
Methods
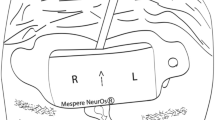
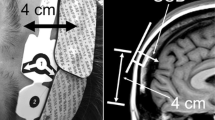
The Institutional Review Board of NorthShore University HealthSystem (Evanston, IL, USA) approved this prospective observational crossover study in February 2014. Written informed consent was obtained from all subjects. Inclusion criteria included subjects who were American Society of Anesthesiologists physical status I, aged 18-45 yr, and with no history of hypertension, diabetes, or neurologic or cardiac disease. This study was conducted in 20 awake healthy volunteers in the supine position and breathing room air. Standard intraoperative monitoring was applied, including an electrocardiogram, pulse oximetry on the left hand, and a manual noninvasive blood pressure cuff placed on the right upper extremity (cycled every two minutes). After cleansing the skin, a Nellcor Max-Fast™ scalp reflectance pulse oximeter (Covidien, Boulder, CO, USA) was placed on the right side of the subject’s forehead. (Fig. 1) The INVOS 5100C and FORE-SIGHT ELITE probes were placed on the left side of the forehead in a random sequence using a computer-generated randomization code. The individual randomization assignments were concealed in envelopes until the subjects entered the study area, and an envelope was then opened immediately before each procedure. A pneumatic head cuff (width = 4.5 cm) (CAS Medical Systems Inc, Branford, CT, USA) was then placed around the subject’s head and secured with duct tape (Fig. 1). The cuff was positioned just below the scalp pulse oximeter and the NIRS sensors and above the supraorbital prominence to reduce the risk of cuff movement during inflation. A study coordinator ensured that the cuff did not impinge on the probes during the study. Accordingly, the cuff could be inflated to produce cutaneous tissue ischemia beneath both probes.
This figure depicts the investigational setup. The scalp pulse oximeter sensor was placed on the right side of the forehead and the cerebral oximeter sensor was placed on the left side of the forehead. The pneumatic cuff was placed below both of these sensors to ensure that they were not compressed during inflation
After a two-minute period of stabilization, baseline heart rate, oxygen saturation (SpO2) –by both peripheral and scalp oximeters, and blood pressure were recorded. In addition, baseline SctO2 was recorded by averaging values every ten seconds over a one-minute period before cuff inflation. The pneumatic head cuff was then inflated to a pressure ≥ 20 mmHg above the mean arterial pressure (MAP). In order to ensure that the cuff pressure remained above these pressure limits, the pressure within the cuff was monitored continuously using the IntelliVue MP90 monitor (Philips Healthcare, Eindhoven, The Netherlands) with a Transpac® IV pressure transducer (Transpac IV monitoring kit 42644-06; ICU medical; San Clemente, CA, USA) attached to the inflation port of the head cuff. Cessation of blood flow to the scalp (i.e., superficial extracranial tissues) was confirmed in all subjects by loss of the photoplethysmogram (PPG) signal from the scalp pulse oximeter. The pneumatic head cuff then remained inflated for five minutes, and SctO2 values were recorded at ten-second intervals. Heart rate, blood pressure, and peripheral and scalp SpO2 values were recorded each minute during cuff inflation. After five minutes of inflation, the head cuff was deflated, and all SctO2 measurements were repeated at ten-second intervals for the next five minutes. After a five-minute deflation interval, the cycle was repeated after the other randomized NIRS probe was placed (i.e., two five-minute inflation measurements and two five-minute deflation measurements were performed).
The primary endpoint measurements were made at two, three, and five minutes post inflation and included the percentage reduction in SctO2 from baseline to these times for the two study devices. We chose these endpoints as they were similar to those of a previous study focusing on extracranial contamination.1 The study coordinator manually transcribed all data from both NIRS monitors and from the Phillips monitor on a case report form.
Statistical analysis
For convenience, the sample size was chosen to be similar to that of a previously published sample study addressing extracranial contamination.1 Data are presented as mean and standard deviation (SD) or median and range/interquartile range [IQR] where appropriate. We applied the Holm-Sidak method for pairwise multiple comparisons used for post hoc analysis (SigmaPlot 11.0, Systat Software, Inc., San Jose, CA, USA) to compare the primary endpoint of SctO2 within and between the different device groups using a two-factor analysis of variance with repeated measures on both factors. The percent decrease from baseline was compared between devices at the same measurement times using the Wilcoxon signed-rank test, and the Bonferroni correction (StatsDirect Statistical Software, Version 2.8.0, Cheshire WA14 4QA, UK) was used to correct the criterion for rejection of the null hypothesis for multiple applications of the test to the same data. The criterion for rejection of the null hypothesis was a two-tailed P < 0.05.
Results
All 20 (eight females, twelve males) volunteer subjects completed the study. Their median (range) age was 19 (18-45) yr. Four of the subjects were African American with darker pigmented skin, and all remaining subjects were Caucasian. Their mean (SD) weight was 69 (14) kg. None of the subjects were on any medication. The SpO2 PPG signal was eliminated upon inflation of the pneumatic cuff in all patients. Heart rate, blood pressure, and SpO2 were similar to baseline and similar between the groups during the inflation and deflation periods (Table 1).
At baseline, there was no difference in SctO2 values between the NIRS devices. During pneumatic cuff inflation, a significant decrease in SctO2 values was observed at two, three, and five minutes for both devices tested. The FORE-SIGHT ELITE device reported significantly lower reductions in SctO2 values at two, three, and five minutes compared with the INVOS 5100C device (Table 2, Fig. 2).
Cerebral oxygen saturation (SctO2, %) determined by INVOS and FORSIGHT monitors before, during (5-min inflation), and after (5-min deflation) inflation of a head tourniquet to induce extracranial tissue hypoxia in 20 subjects. The lower boundary of each box indicates the 25th percentile, the line within each box indicates the median, and the upper boundary of each box indicates the 75th percentile. Whiskers above and below each box indicate the 90th and 10th percentiles. Outlying points are graphed above and below the upper and lower whiskers, respectively. The five-minute inflation measurements differed from their respective baselines and from each other as indicated by *
The smaller relative change from baseline reported by the FORE-SIGHT ELITE monitor after two, three, and five minutes of cuff inflation was statistically significant when compared with that recorded by the INVOS device (Table 3). After five minutes of cuff inflation, the INVOS 5100C recorded a median [IQR] decrease in SctO2 from baseline of 15.1% [12.6 -17.6], while that recorded at the same time by the FORE-SIGHT ELITE device was 8.6% [4.0 -12.3] (median difference, 7.9%; 99% confidence interval, 1.9 to 16.5; P = 0.002) (Table 3). The relative median [IQR] change from baseline reported by the INVOS device after five minutes of deflation was 0.0% [-2.9 - 2.4]. The relative median [IQR] change from baseline reported by the FORE-SIGHT ELITE monitor at the same time was 0.2% [-3.5 - 3.1]. There were no significant differences in SctO2 values between the devices after deflation (Table 3).
Discussion
This investigation in volunteers suggests that, although extracranial contamination remained present in both devices as indicated by a drop in SctO2 values during scalp ischemia, significantly less contamination was recorded with the second-generation FORE-SIGHT ELITE cerebral oximeter device than with the INVOS 5100C device. Additional wavelengths of light as well as updated and changed algorithms15 could account for this reduction in the observed amount of extracranial contamination with the newer generation cerebral oximeter.
In order to ensure that only the oxygenation status of hemoglobin in the brain is measured, the probes on NIRS devices typically use two light detectors located at fixed distances from the light source. The depth of the light penetration is proportional to the distance from the emitting light source to the receiving light detector.1–6 The near-field light detector, which is located closest to the light source, measures signals primarily within the extracranial tissues, while the far-field detector measures both cerebral and extracranial tissue saturation.1–6 Though the exact algorithm for each of these devices is proprietary, both are spatially resolved spectrometers.1–6
The INVOS 5100C used in this study uses two wavelengths of infrared light (730 and 810 nm) and two light detectors at two different fixed distances (proximal and distal) from the light source.1,9–12 The second-generation FORE-SIGHT ELITE device used in this study uses an enhanced proprietary algorithm coupled with five wavelengths of infrared light (680, 730, 770, 805, and 870 nm) to improve the accuracy of the blood oxygenation measurements.14 MacLeod et al. examined the accuracy of the first- and second-generation FORE-SIGHT monitors in volunteers.15 Arterial and jugular venous bulb oxygen concentrations were measured and compared with simultaneously measured SctO2 values during alterations in blood oxygen and carbon dioxide concentrations. The investigators observed that the FORE-SIGHT ELITE measured SctO2 with greater accuracy than the first-generation FORE-SIGHTdevice.15 It has yet to be determined if the FORE-SIGHT ELITE, with its technological advances, can achieve a greater reduction in extracranial contamination than the first-generation monitor.15
A few studies have specifically examined the important issue of extracranial contamination. A prior study using an earlier version of the INVOS (3100) device revealed that inflation of a scalp tourniquet, which affects extracranial blood flow and oxygenation, also affected SctO2 values.13 In a different study, Davie et al. examined the influence of extracranial contamination on SctO2 measurements made with three different cerebral oximetry technologies.1 Twelve volunteers had each of three NIRS devices applied to their forehead (INVOS 5100C, FORE-SIGHT, and EQUANOX™ [Nonin Medical Inc, Plymouth, MN, USA]). A tourniquet similar to our own was then placed around each volunteer’s head (below the level of the NIRS probes) to induce ischemia in the tissue below the NIRS probes when inflated. An evaluation was then carried out on the effect of cuff inflation and resulting extracranial tissue contamination on the SctO2 values from all three devices. The authors observed that the induction of extracranial ischemia for five minutes resulted in significant mean reductions in SctO2 from baseline values in all three devices (INVOS 5100C 16.6%, FORE-SIGHT 11.8%, and EQUINOX 6.8%).1
In the present study, the INVOS was shown to have a similar mean (reported here for the sake of comparison with the previous study) relative decrease in SctO2 values from baseline at five minutes after cuff inflation (16.1% relative decrease). Nevertheless, the FORE-SIGHT ELITE appeared to have a smaller mean reduction from baseline than the previous model studied by Davie et al. (7.3% vs 11.8%, respectively).
There are several plausible reasons why the FORE-SIGHT ELITE device recorded less extracranial contamination than the original FORE-SIGHT device studied by Davie et al. First, the new device uses five wavelengths (680, 730, 770, 805, and 870 nm) vs four wavelengths (690, 780, 805, and 850 nm) used in the first-generation device, with nearly equidistant spacing over the lower saturation range.15 These modifications, coupled with a changed proprietary computer algorithm, may have compensated for extracranial tissue interference.15 Second, the present study monitored the pneumatic cuff pressure by transducing the pressure throughout the inflation period. This was done to ensure that the pneumatic cuff pressure was sustained at a stable and high enough level to facilitate continuous ablation of the SpO2 PPG signal and to allow static pressure to be applied to the forehead. The study by Davie et al. did not continuously measure pneumatic cuff pressure, and therefore, the pressure they applied may have differed throughout the inflation period. In addition, we increased cuff pressure to ≥ 20 mmHg above MAP, while Davie et al. increased the cuff pressure to above systolic blood pressure (SBP). Before we initiated this study, we found that the SpO2 PPG signal was consistently suppressed when using the cuff pressure threshold of ≥ 20 mmHg above MAP. Furthermore, using a cuff pressure of ≥ 20 mmHg above SBP did not consistently suppress the SpO2 signal. It also appeared that the healthy volunteers experienced more pain (data not shown) with the SBP cut-off than with the MAP cut-off, which is why we used the latter measurement. It is still unclear how this may have ultimately affected the present results.
The clinical significance of extracranial contamination remains unknown. Different studies use a variety of cerebral oximetry endpoints in an attempt to affect clinical decision-making. Some suggest that the relative change in SctO2 values from baseline might be a more suitable measurement to use for management decisions than absolute thresholds. Patients with a low starting SctO2 value may require more changes in therapeutic strategy despite small relative changes in SctO2 values from baseline because their starting values are closer to the critical threshold value for treatment. Moreover, using the relative change from baseline strategy may also be subject to inaccuracies depending on the degree of change in the clinical situation (e.g., during cardiopulmonary bypass, significant hemodynamic changes, or use of vasoconstrictive agents such as phenylephrine, etc.). Studies have shown that, when phenylephrine is administered to patients undergoing general anesthesia, SctO2 values drop significantly compared with when ephedrine is administered.16 While authors propose that this may be due to changes in cardiac output, phenylephrine may also cause cutaneous vaso/venoconstriction that is expressed as an SctO2 reduction.16 Another study by Sorenson et al. examined 50 healthy males under different physiologic conditions. The authors suggested that both norepinephrine and hyperthermia affected skin oxygenation and subsequently influenced SctO2 readings by reducing the values.17
There are no regulatory standards thus far with respect to error in the precision of cerebral oximetry readings. Nevertheless, when examining the pulse oximeter, a monitor similar in design to a cerebral oximeter, the regulatory standards permit SpO2 values to vary from baseline up to 3.5% according to the U.S. Food and Drug Administration and up to 4% according to the International Organization for Standardization.18,19 The present FORE-SIGHT ELITE monitor recorded values that were less than two standard deviations from baseline (7.3%), which is likely to be clinically acceptable. The extreme conditions of induced cutaneous ischemia from pneumatic cuff compression may not be reproducible in real-time clinical settings; therefore, this deviation may even be less during other clinical conditions.
There are several limitations of the present study. First, the assumption that the cerebral oxygen saturation is a value that reflects a mixture of venous (70%) and arterial (30%) blood in the frontal cerebral cortex may not always hold true in different populations as well as under different physiologic conditions. Ito et al. suggested that changes in cerebral blood volume during hyper- or hypocapnia were largely due to changes in arterial blood volume and not venous or capillary blood volume.20 These findings may hold true during the extreme cutaneous ischemia induced by pneumatic cuff inflation, making the SctO2 difficult to interpret. Second, this study examined a small cohort of healthy volunteers. Cerebral oximetry readings may be significantly different in patients with significant comorbidities in real-time dynamic clinical settings. Third, all subjects were placed in the supine position, and therefore, the extent that cutaneous ischemia may differ in other positions (i.e., beach chair or prone) is unknown. Fourth, the small number of volunteers in this study may not lend itself to broad conclusions; however, this volunteer study included nearly twice as many subjects as the previous study by Davie et al.1 Fifth, other devices that may measure cerebral oxygenation more directly (e.g., jugular bulb venous saturation and microdialysis catheters) were not used in this study.3,4 The study did not determine the effect of ischemia induced by pneumatic cuff inflation on the measurements made by jugular bulb venous saturation and microdialysis catheters or compare their measurements with those of the present devices.
In addition, the sample size was insufficient for a statistical evaluation of the effect of volunteer skin pigmentation and forehead shape on SctO2 measurements. Four subjects were African American with darker pigmented skin than the others; however, skin pigmentation did not appear to affect baseline, inflation, or deflation measurements.
In summary, this study compared cerebral oximeter measurements by the INVOS device (previously studied by Davie et al.) with measurements by the updated FORE-SIGHT ELITE device after pneumatic cuff inflation. The results suggest that both devices are subject to extracranial contamination. Nevertheless, the FORE-SIGHT ELITE device was significantly less affected by extracranial contamination than the INVOS device. The effect of this extracranial contamination on a variety of clinical conditions and on the practical use of these devices remains unclear.
Notes
Personal communication, Robert Kopotic, CAS Medical Systems Inc.
References
Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies. Anesthesiology 2012; 116: 834-40.
Zheng F, Sheinberg R, Yee MS, Ono M, Zheng Y, Hogue CW. Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: a systematic review. Anesth Analg 2013; 116: 663-76.
Rao GS, Durga P. Changing trends in monitoring brain ischemia: from intracranial pressure to cerebral oximetry. Current Opinion Anaesthesiol 2011; 24: 487-94.
Calderon-Arnulphi M, Alaraj A, Slavin KV. Near infrared technology in neuroscience: past, present, and future. Neurol Res 2009; 31: 605-14.
Fischer GW. Recent advances in application of cerebral oximetry in adult cardiovascular surgery. Semin Cardiothorac Vasc Anesth 2008; 12: 60-9.
Matcher SJ, Kikpatrick PJ, Nahid K, Cope M, Delpy DT. Absolute quantification methods in tissue near infrared spectroscopy. SPIE Proceedings 1995; 2389: 486-95.
Slater JP, Guarino T, Stack J, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg 2009; 87: 36-44.
Casati A, Fanelli G, Pietropaoli P, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg 2005; 101: 740-7.
Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg 2010; 111: 496-505.
Goldman S, Sutter F, Ferdinand F, Trace C. Optimizing intraoperative cerebral oxygen delivery using noninvasive cerebral oximetry decreases the incidence of stroke for cardiac surgical patients. Heart Surg Forum 2004; 7: E376-81.
Murkin JM, Adams SJ, Novick RJ, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg 2007; 104: 51-8.
Fischer GW, Silvay G. Cerebral oximetry in cardiac and major vascular surgery. HSR Proc Intensive Care Cardiovasc Anesth 2010; 2: 249-56.
Germon TJ, Young AE, Manara AR, Nelson RJ. Extracerebral absorption of near infrared light influences the detection of increased cerebral oxygenation monitored by near infrared spectroscopy. J Neurol Neurosurg Psychiatry 1995; 58: 477-9.
Ikeda K, MacLeod DB, Grocott HP, Moretti EW, Ames W, Vacchiano C. The accuracy of a near-infrared spectroscopy cerebral oximetry device and its potential value for estimating jugular venous oxygen saturation. Anesth Analg 2014; 119: 1381-92.
MacLeod D, Ikeda K, Cheng C, Shaw C. Validation of the next generation FORE-SIGHT Elite tissue oximeters for adult cerebral tissue oxygen saturation. Anesth Analg 2013; 116 (Supp):1-182 (abstract).
Meng L, Cannesson M, Alexander BS, et al. Effect of phenylephrine and ephedrine bolus treatment on cerebral oxygenation on anaesthetized patients. Br J Anaesth 2011; 107: 209-17.
Sorensen H, Secher NH, Siebenmann C, et al. Cutaneous vasoconstriction affects near-infrared spectroscopy determined cerebral oxygen saturation during administration of norepinephrine. Anesthesiology 2012; 117: 263-70.
Pulse Oximeters - Premarket Notification Submissions [510(k)s]: Guidance for Industry and Food and Drug Administration Staff. Issued on: March 4, 2013. Available from URL: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM081352.pdf (accessed July 2015).
International Organization for Standardization. ISO 80601-2-61:2011. Medical Electrical Equipment – Part 2-61: Particular Requirements for Basic Safety and Essential Performance of Pulse Oximeter Equipment. Available from URL: http://www.iso.org/iso/catalogue_detail.htm?csnumber=51847 (accessed July 2015).
Ito H, Ibaraki M, Kanno I, Fukuda H, Miura S. Changes in the arterial fraction of human cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab 2005; 25: 852-7.
Conflicts of interest
Both Drs. Greenberg and Murphy have served in the past as consultants for CASMED, the developer of the FORE-SIGHT ELITE cerebral oximeter. CASMED provided all cerebral oximeter monitors and probes for this study but had no role in the study conduct, data analysis, or input into manuscript preparation
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Steven Greenberg and Glenn Murphy contributed substantially to all aspects of this manuscript and drafted the article. Steven Greenberg, Glenn Murphy, Torin Shear, Joseph Szokol, and Jeffery Vender contributed substantially to the conception and design of the manuscript. Steven Greenberg, Glenn Murphy, Aashka Patel, and Andrew Simpson contributed substantially to the acquisition of the data. Michael J. Avram contributed substantially to the analysis and interpretation of the data.
Rights and permissions
About this article
Cite this article
Greenberg, S., Murphy, G., Shear, T. et al. Extracranial contamination in the INVOS 5100C versus the FORE-SIGHT ELITE cerebral oximeter: a prospective observational crossover study in volunteers. Can J Anesth/J Can Anesth 63, 24–30 (2016). https://doi.org/10.1007/s12630-015-0451-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-015-0451-7