Abstract
Purpose
Hemodilution in perioperative patients has been associated with neurological morbidity and increased mortality by undefined mechanisms. This study assesses whether hemodilutional anemia up-regulated inflammatory cerebral gene expression (microarray) to help define the mechanism.
Methods
Hemodilution was performed in anesthetized rats by exchanging 50% of the estimated blood volume (30 mL kg−1) with pentastarch. Two groups of control animals were utilized, i.e., a non-anesthetized control (Normal Control) and an anesthetized control group (Anesthesia Control). Blood pressure, hemoglobin concentration, and arterial blood gas analysis were performed before and after hemodilution. Cerebral cortex was harvested from isoflurane-anesthetized rats (n = 6) after 6 and 24 hr of recovery and was used to perform complimentary DNA (cDNA) microarray analyses. Pro-inflammatory chemokine and cytokine protein levels were also measured.
Results
Microarray analysis demonstrated up-regulation of 72 and 27 genes (6 and 24 hr, respectively) in anemic cerebral cortex. These genes were involved in a number of biological functions, including (1) inflammatory responses; (2) angiogenesis; (3) vascular homeostasis; (4) cellular biology; and (5) apoptosis. Chemokine ribonucleic acid (RNA) expression (CXCL-1, -10, and -11) was highest in anemic brain tissue (P < 0.0125 for each). Protein measurements demonstrated a significant increase in interleukin-6, tumor necrosis factor α, and monocyte chemoattractant protein-1 (P < 0.05 for each).
Conclusion
This study utilizes microarray technology to elucidate changes in cerebral cortical gene expression in response to acute hemodilution. These findings demonstrate an increase in pro-inflammatory chemokines (RNA, protein) and cytokines (protein). An improved understanding of the inflammatory response to anemia may help to minimize associated morbidity and mortality.
Résumé
Objectif
L’hémodilution en périopératoire chez les patients a été associée à une morbidité neurologique et une mortalité accrue en raison de mécanismes indéterminés. Cette étude examine si l’anémie par hémodilution régule à la hausse l’expression génique cérébrale inflammatoire (microarray) afin d’aider à définir ce mécanisme.
Méthode
Une hémodilution a été réalisée chez des rats anesthésiés en échangeant 50 % du volume sanguin estimé (30 mL·kg−1) avec du pentastarch. Deux groupes d’animaux témoins ont été utilisés, soit un groupe témoin non anesthésié (témoin normal) et un groupe témoin anesthésié (témoin anesthésié). La pression artérielle, la concentration d’hémoglobine et une gazométrie sanguine artérielle ont été réalisées avant et après hémodilution. Le cortex cérébral a été récolté chez les rats anesthésiés à l’isoflurane (n = 6) après 6 et 24 h de récupération et a été utilisé pour réaliser des analyses par microarray d’ADN complémentaire (ADNc). Les niveaux des protéines pro-inflammatoires chimiokine et cytokine ont également été mesurés.
Résultats
L’analyse par microarray a démontré une régulation à la hausse de 72 et 27 gènes (6 et 24 h, respectivement) dans le cortex cérébral anémique. Ces gènes étaient impliqués dans plusieurs fonctions biologiques, notamment 1) les réactions inflammatoires; 2) l’angiogenèse; 3) l’homéostasie vasculaire; 4) la biologie cellulaire; et 5) l’apoptose. L’expression de l’acide ribonucléique (ARN) des chimiokines (CXCL-1, 10 et 11) était la plus élevée dans le tissu cérébral anémique (P < 0,0125 pour chacune). Les mesures des protéines ont démontré une augmentation significative de l’interleukine-6, du facteur onconécrosant α, et de la protéine MCP1 (P < 0,05 pour chacun).
Conclusion
Cette étude utilise la technique du microarray pour expliquer les changements dans l’expression génique du cortex cérébral en réaction à une hémodilution aiguë. Ces résultats démontrent une augmentation des chimiokines (ARN, protéines) et des cytokines (protéines) pro-inflammatoires. Une meilleure compréhension de la réaction inflammatoire à l’anémie pourrait nous aider à minimiser la morbidité et la mortalité associées.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute anemia has been associated with cognitive and neurophysiologic dysfunction in healthy volunteers1,2 and increased neurological injury and mortality in perioperative patients by currently undefined mechanisms.3–6 One of the most common causes of acute anemia is acute blood loss and fluid resuscitation (hemodilution). Therefore, we have adopted a model of acute hemodilutional anemia to study the cerebral gene responses. Experimental evidence suggests that hemodilution may augment cerebral injury by limiting cerebral tissue oxygen delivery.7–12 However, the mechanism by which cerebral injury occurs in anemic patients has not been clearly defined.
Our research has demonstrated that acute hemodilutional anemia leads to the up-regulation of a number of proteins in the brain, including hypoxia inducible factor-1α (HIF-1α) and neuronal and inducible nitric oxide synthase (nNOS and iNOS, respectively).8,12 However, the role of these or other proteins in the pathophysiology of anemia-induced brain injury has not been defined.
Previous studies have identified that acute hemodilution can increase the migration of leukocytes into the central nervous system.9 In addition, our published experimental study demonstrated an increase in cerebral cortical iNOS expression, which is suggestive of a pro-inflammatory response.8 Interestingly, this increase in iNOS expression was not associated with an increase in circulating cytokines, as plasma tumor necrosis factor (TNF)α, interleukin (IL)-6, and IL-1β levels did not differ between the control and anemic groups.8 To assess whether the increase in cerebral cortical iNOS expression was the result of a local tissue inflammatory response, we performed a microarray analysis to test the hypothesis that acute inflammation could contribute to cerebral injury associated with acute hemodilutional anemia.
In addition, the discovery of other novel genes that are up-regulated by acute hemodilution may provide important insights into adaptive and maladaptive mechanisms activated in the cerebral cortex in response to anemia. These data may provide clues to potential target genes for preventing cerebral injury and optimizing the treatment of anemic patients.
Materials and methods
Animal model
All animal protocols were approved by the Animal Care and Use Committee at St. Michael’s Hospital in accordance with the requirements of Canadian Animal Care. Anesthesia was induced in male Sprague–Dawley rats (Charles River, St. Constant, QC, Canada) with 3–4% isoflurane in 100% oxygen in an induction chamber and maintained with 1–2% isoflurane in 50% oxygen (Abbott, St.Laurent, QC, Canada). Following intubation, ventilation was maintained with a pressure-controlled ventilator (Kent Scientific, Litchfield, CT, USA). Incision sites were infiltrated with 2% lidocaine prior to cannulation of the tail artery and vein (24 G BD Angiocath™, BD Medical Systems, Oakville, ON, Canada). Vascular access was utilized to measure mean arterial blood pressure (MAP), arterial blood gases (ABGs), and hemoglobin concentration by co-oximetry (Radiometer ALB 500 and OSM 3 London Scientific, London, ON, Canada) and to perform acute hemodilution. Ventilation was adjusted to achieve normocapnia and normoxia as determined by blood gas analysis. A heating pad and heating lamp were used to maintain rectal temperature near 37°C. Data were recorded using a computerized data acquisition system (DASYLab 5.6, Kent Scientific, Litchfield, CT, USA).
After establishing baseline ABGs and hemoglobin concentrations, acute hemodilutional anemia was induced by simultaneously exchanging 30 mL · kg−1 of arterial blood (50% of the estimated blood volume) withdrawn from the tail artery with an equivalent volume of pentastarch (Pentaspan, Bristol-Myers Squibb, Montreal, QC, Canada) infused via the tail vein (n = 6 rats per time period) (Anemia Group). Volume exchange was performed over 10 min using a programmable “push–pull” pump as previously described (PHD 2000, Harvard Apparatus, Saint-Laurent, QC, Canada).10 This model of acute hemodilution approximates clinical conditions of hemodilution that commonly occur during cardiopulmonary bypass or fluid resuscitation with crystalloid and/or colloid solution prior to utilization of blood transfusion. Following completion of volume exchange, all parameters were recorded for an additional 20 min. The rats were then recovered until they were breathing room air spontaneously. Two groups of control animals were utilized. One group was briefly anesthetized while spontaneously breathing 2% isoflurane in 50% oxygen prior to blood sampling by aortic puncture (room air), decapitation, and brain harvest (Normal Control). The second group served as a procedural control similar to the hemodilution group with respect to all interventions, including anesthetic and cannulation technique, but did not undergo hemodilution (Anesthesia Control). The two different groups of control anesthetized and hemodiluted rats were recovered for either 6 or 24 hr prior to brain harvest. At 6 or 24 hr, the rats were anesthetized with 2% isoflurane in 50% oxygen and then decapitated. The brains were harvested and segments of cerebral cortex were snap frozen in liquid nitrogen (−80°C) within 4 min. RNA extraction and microarray analysis were performed as outlined below.
Microarray analysis
High-density cDNA microarrays (GeneChip® Rat Genome 230 2.0 Array, of 31,000 well-substantiated rat genes, Affymetrix®, Santa Clara, CA, USA) were used to examine genes whose expressions are regulated following acute hemodilution. To define the transcriptomes of anemia, RNA samples were prepared from the cerebral cortex of rats 6 and 24 hr after hemodilution. The samples were compared with RNA from animals that had previously received sham treatment consisting of anesthesia and surgical procedures without hemodilution (Anesthesia Control) and from animals immediately killed under brief general anesthesia (Normal Control). The cerebral cortex from two rats for each experimental condition were pooled (three pools per condition) to minimize potential variation among animals. Predefined criteria were set to increase the level of confidence in the analysis of the microarray data.11 These criteria were as follows: (1) filtering genes of interest using a confidence interval of 0.0125; (2) correcting the one-way analysis of variance (ANOVA) using the Benjamini and Hochberg method to control the false discovery rate; and (3) presenting only those genes with a two-fold change from Normal Controls.
As outlined below, samples of RNA were prepared for microarray analysis, which was performed by the Microarray Facility at the Centre for Applied Genomics, Hospital for Sick Children (Toronto, ON, Canada).
RNA preparation
Total RNA was isolated from the snap-frozen cerebral cortex tissue using QIAzol Lysis reagent (Qiagen, Mississauga, ON, Canada) according to the manufacturer’s protocol. After isolation, the RNA was purified using the RNeasy total RNA isolation kit (Qiagen). RNA samples were quantified spectrophotometrically, and samples with a ratio A260/A280 from 1.8 to 2.1 were analyzed for quality using a bioanalyser (Agilent Technologies, Palo Alto, CA, USA). Equal amounts of total RNA preparation from two individual animals in each group (Anemia 6 and 24 hr, Anesthesia Control 6 and 24 hr, and Normal Control 0 hr) were pooled (n = 3 arrays, n = 6 rats per group and time) and used as a starting material for each microarray template reaction.
Synthesis of complimentary DNA (cDNA)
The Affymetrix® protocol for GeneChip® one-cycle cDNA synthesis was used. Briefly, the reaction was primed by annealing a T7-oligo (dT) primer coupled to a T7 RNA polymerase promoter to the RNA sample. RNA was reverse transcribed with Superscript II™ reverse transcriptase. Second-strand synthesis was carried out immediately in the presence of Escherichia coli DNA polymerase I with RNAse H, incubated in the presence of T4 DNA polymerase, and terminated with ethylenediamine tetraacetic acid (EDTA). The samples were cleaned (Qiagen cDNA clean-up column).
Synthesis of biotin-labeled complementary RNA (cRNA)
Complementary RNA was generated in an in vitro transcription reaction by using T7 RNA polymerase and biotinylated ribonucleotide analog (IVT labelling kit, Affymetrix®). The in vitro transcription samples were cleaned (Qiagen RNA clean-up column) and eluted. The quality was confirmed spectrophotometrically (260/280).
Fragmentation and hybridization to species microarray
Twenty microgram of each resulting biotinylated cRNA were fragmented into strands of 35–200 bases in length according to the manufacturer’s protocol (Affymetrix GeneChip® analysis technical manual) and added to the hybridization buffer. Eukaryotic hybridization controls (BioB, BioC, BioD, and cre) and control oligonucleotides were added to the cocktail. The hybridization cocktail was denatured (99°C for 5 min). The Affymetrix® chip arrays (rat 230 2.0 array) were equilibrated to room temperature. Sample cocktails were introduced into the chamber of the preconditioned chips and incubated (45°C for 16 hr). The chips were washed, stained, and scanned using a fluidic station 400 and an Affymetrix GeneChip® Scanner 3000.
Protein analysis
After analysis of the microarray data at 6 hr, maximal responses to inflammatory chemokines were identified. Hemodilution or anesthesia control was performed on additional groups of rats (n = 6 for each). Brain tissue was harvested to assess changes in chemokine and cytokine protein levels using the Procarta cytokine kit (PC4110, Panomics Inc., Fremont, CA, USA) and Luminex 100 detection unit.
Statistical analysis
Physiological data
Statistical analysis was performed using SigmaStat (V2.03S, Systat Software Inc., Pint Richmond CA, USA). Data were assessed by a one or two-way ANOVA. For two-way ANOVA, significant time, group, and interaction effects were identified. When a significant F value was observed, post hoc analysis was performed using a Tukey test. Data are reported as mean ± SD. P < 0.05 was assessed as significant.
Microarray data
The Affymetrix GeneChip® system was used to convert intensity data into quantitative estimates of gene expression for each probe set (which includes 16 probe pairs consisting of a perfect match oligonucleotide and a mismatch oligonucleotide that contains a one-base mismatch at a central position). A probability statistic was generated for each probe set to define the genes that were present, marginal, or absent after background subtraction. Global scaling expression data were analyzed using GeneSpring software (Silicon Genetics, Redwood City, CA, USA). The data for both treatments (Anemia and Anesthesia Control) and the two times (6 and 24 hr) were normalized to Normal Control (0 hr). The data were filtered to decrease the number of irrelevant unchanging genes.11 One filter was designed to identify genes that are present and/or marginal in at least 2–3 arrays for each treatment and time. The second filter was designed to identify genes that were significantly up- or down-regulated compared with Normal Control by using a confidence interval of 0.0125 for at least one condition for each time point. After filtering, one-way ANOVA with Benjamini and Hochberg correction was performed and twofold up- or down-regulated genes in each time point for both treatments were used for ontology analysis and pathway construction.
Chemokine and cytokine protein data
Cerebral cortical chemokine and cytokine levels were assessed by one-way ANOVA.
Results
Physiological data
The MAP of the anesthesia control and anemia groups was not significantly different either before (69 ± 10 vs 65 ± 7 mmHg, respectively) or after hemodilution (72 ± 8 vs 75 ± 4 mmHg, respectively). Baseline hemoglobin values did not differ between anesthesia controls and rats about to undergo hemodilution (120–130 g · L−1) (Table 1). Following hemodilution, there was a significant reduction in the hemoglobin concentration to values approximating 60 g · L−1 (Table 1). No differences in any other co-oximetry or ABG measurement were observed between the anesthetized controls and the hemodilution group.
Microarray data
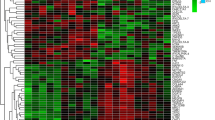
Microarray analyses demonstrated that 18,000 out of 31,000 genes passed the first filter step for both time points. After the second filter, 435 and 217 genes met the second criterion at 6 and 24 hr, respectively. At 6 hr, 131 of these genes demonstrated differential regulation for both conditions (Anemia and Anesthesia Control) when compared with the cerebral cortex from the non-intervention controls. At 24 hr, only 33 genes showed differential regulation in both groups. None of the genes reported demonstrated greater than twofold down-regulation at either 6 or 24 hr.
Gene ontology, a method of classifying expressed genes by their reported or suggested molecular function, was utilized to group genes according to function. This was achieved by a multimodal approach utilizing information from the program, GeneSifter™ (www.genesifter.net), the universal protein resource (www.expasy.uniprot.org), and a literature review. Some genes may fall into more than one functional category. Utilizing these categories helps to assess the large amount of data acquired (Tables 2 and 3). Pathway construction based on gene expression data was performed using Pathway Architect (www.stratagene.com). In the anemia group, 89% and 90% of the defined genes showed at least a twofold change in expression at 6 and 24 hr, respectively. In the anesthesia group, 72% and 46% of the defined genes showed at least a two-fold change in expression at 6 and 24 hr, respectively. Of these genes up-regulated by anemia, 72 and 27 genes had a known function at 6 and 24 hr, respectively. For the anesthesia controls, 58 and 14 genes had known functions at 6 and 24 hr, respectively.
After 6 hr of anemia, up-regulation of cerebral cortical gene expression occurred in a number of important biological areas: (1) immune and inflammatory response genes (26% of all filtered genes); (2) regulation of transcription/translation and protein biology (10%); (3) apoptosis (9%); (4) angiogenesis (7%); (5) cellular biology (7%); (6) signal transduction (6%); (7) vascular homeostasis (5%); and (8) ion transport (3.7%). After 24 hr of anemia, changes in gene expression still occurred in: (1) immune and inflammatory pathways (32% of all filtered genes); (2) cellular biology (24%); (3) vascular biology (12%); (4) protein biology (8%); and (5) angiogenesis, metabolism, ion transport, and signal transduction (4%, respectively) (Fig. 1). There was no evidence of up-regulation of genes involved in apoptosis and regulation of transcription/translation after 24 hr of anemia (Fig. 1). A particular common pattern of gene expression occurred when comparing the gene responses with the Anemia and Anesthesia Controls. However, the number of genes specifically up-regulated by anemia was larger at 6 hr in the following areas: (1) immune and inflammatory response; (2) vascular homeostasis; (3) cellular biology; and (4) signal transduction (Fig. 1, Upper panels) and larger at 24 hr in the following areas: (1) immune and inflammatory response; (2) angiogenesis; and (3) ion transport (Fig. 1, Upper and middle panels).
Genes showing a twofold change in the cerebral cortex representing the different biological processes defined using GeneSifter™ software at 6 and 24 hr after acute hemodilutional anemia (Anemia) and anesthesia + surgical stress (Anesthesia Control) at 6 and 24 hr in the cerebral cortex for the different biological processes defined using GeneSifter software
Specific changes in individual genes are outlined in Tables 2 and 3. Different genes were defined for each functional group in anemic and anesthesia controls. When compared with controls at 6 hr, the most striking fold change differences observed among the gene profile in response to anemia were: (1) immune and inflammatory responses (chemokine [C–X–C motif] ligands 1, 10, and 11 [CXCL-1,10,11]; guanylate nucleotide binding protein 2; and interferon-induced protein with tetratricopeptide repeats 2: 66.0-, 35.0-, 23.1-, 16.6-, and 11.9-fold, respectively); (2) vascular homeostasis (prostaglandin E synthase: 64.5-fold); (3) apoptosis (cyclin-dependent kinase inhibitor 1A and lipocalin 2: 63.1- and 36.1-fold, respectively); and (4) cellular biology (uridine phosphorylase 1, S100 calcium binding protein A9, and metallothionein 1a: 41.9-, 18.8-, and 14.1-fold, respectively). Of these genes, only S100 calcium binding protein A9 remained significantly elevated at 24 hr in both conditions (lower for Anemia [18.5-fold] than for Anesthesia Control [26-fold]). In contrast, S100 calcium binding protein A8 was only up-regulated at 24-hr and at the same level in both conditions (25-fold). Additional cerebral cortical genes that were increased after acute anemia included programmed cell death 1 ligand 2 (6.9-fold at 6 hr), CCAAT/enhancer binding protein (C/EBP), beta (3.8-fold at 6 hr), zinc finger protein 36 (3.1-fold at 6 hr), angiopoeitin 2 (2.6- and 2.8-fold at 6 and 24 hr), thrombospondin 1 (2.9-fold at 6 hr), fibrinogen-like 2 (7.3-fold at 6 hr), albumin (2.1-fold at 6 hr), von Willebrand factor (3.0-fold at 24 hr), annexin A2 (2.1-fold at 24 hr), vimentin (2.5-fold at 24 hr), and palmedelphin (2.1-fold at 24 hr). A number of genes increased in response to anesthesia and surgical stress (Anesthesia Control) at 6 and 24 hr (Tables 2 and 3). Pathway Architect was used to demonstrate the relationship between up-regulated genes after acute anemia. A specific network between several up-regulated genes from a number of different categories was assessed with respect to their relationship with three hypoxia-regulated molecules previously demonstrated to be involved in the cerebral response to acute anemia (hypoxia inducible factor-1α (HIF-1α), erythropoietin (EPO), and vascular endothelial growth factor (VEGF)12 (Fig. 2).
Pathway analysis of the genes showing at least a twofold change at 6 and 24 hr after acute anemia. One network containing 23 genes was defined using Pathway Architect and is displayed graphically as nodes (genes/protein products) and edges (the connections—binding, positive and negative regulation—between the nodes). Three genes involved in response to hypoxia were also included in the analysis: hypoxia inducible factor-1α (HIF-1A), erythropoietin (EPO), and vascular endothelial growth factor (VEGF). ADAMTS1 = thrombospondin 1; ANGPT2 = angiopoietin 2; BID = BH3 interacting domain death agonist; BIRD4 = baculoviral IAP repeat-containing 4; CDKN1A = cyclin-dependent kinase inhibitor 1A; CEBPB = CCAAT/enhancer binding protein (C/EBP) beta; CEBPD = CCAAT/enhancer binding protein (C/EBP) delta; CXCL1 = chemokine ligand 1; CXCL9 = chemokine ligand 9; CXCL10 = chemokine ligand 10; CXCL11 = chemokine ligand 11; FCGR2B = Fc receptor IgG low affinity III; IL2R = interleukin 2 receptor; IL6R = interleukin 6 receptor; IRF1 = interferon regulatory factor 1; MT1A = metallothionein 1a; NFKBIA = nuclear factor of kappa light chain gene enhancer in B-cells inhibitor alpha; PTPN11 = protein tyrosine phosphatase non-receptor type 11; RET = retinal pigment epithelium 65; S100A8 = S100 calcium binding protein A8; S100A9 = S100 calcium binding protein A9; STAT3 = signal transduction and activator of transcription 3; VWF = von Willebrand factor
Protein data
Compared with anesthesia controls, significant increases in cerebral cortical levels of IL-6, TNFα, and monocyte chemoattractant protein (MCP)-1 were measured in anemic brain tissue (P < 0.05 for each, Fig. 3).
Cerebral cortical protein levels in anemic rats demonstrate elevated levels of pro-inflammatory cytokine interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-a), and the chemokine monocyte chemoattractive protein-1 (MCP-1). No increased in intracellular adhesion molecule-1 (ICAM-1) or RANTES (Regulated on Activation, Normal T Expressed and Secreted). * P < 0.001, ** P < 0.05
Discussion
Microarray analysis demonstrated that a significant number of genes underwent up-regulation in response to acute hemodilutional anemia. The most striking increases were observed for the inflammatory chemokines, C–X–C ligand 1, 10, and 11. This pattern of gene expression suggested that pro-inflammatory mechanisms had been activated within the cerebral cortex of anemic rats. Subsequent analysis confirmed that TNFα, IL-6, and MCP-1 protein levels were also up-regulated in cerebral cortical tissue. Up-regulation of these proteins has been associated with increased leukocyte and monocyte migration into the brain.13,14 Local production of these cytokines within the brain (TNFα, IL-6) is strongly supported by the results of our previous study wherein anemia did not increase the levels of circulating cytokines above control values.8 Thus, local up-regulation of inflammatory mediators and subsequent leukocyte migration into the brain may contribute to anemia-induced cerebral injury.
In addition, strong up-regulation of a number of different genes was observed at 6 hr (prostaglandin E synthase, uridine phosphorylase 1 and apoptotic mediators, P-selectin, CCAAT/enhancer binding protein (C/EBP) beta and delta, S100 calcium binding protein A9, and metallothionein 1a). By 24 hr, most of these genes were no longer up-regulated. The two exceptions were S100 calcium binding protein A9 and angiopoietin 2, which remained elevated at 6 and 24 hr. After 24 hr, additional genes demonstrated up-regulation that was not expressed at 6 hr (S100 calcium binding protein A8, von Willebrand factor, and Annexin A2).
Significant changes in gene expression were also caused by surgical and anesthetic procedures (Tables 2 and 3). The magnitude of the changes observed was generally smaller when compared with changes observed after acute hemodilution. It is of interest that none of the genes reported in Tables 2 and 3 exhibited a significant decrease in expression (greater than two-fold). By focusing on the genes that were up-regulated in both groups, we were able to identify those genes whose expression was solely increased because of anemia, thereby excluding the effects of surgical and anesthetic procedures. These data demonstrate a complex biological pattern of cerebral cortical gene expression, which may contribute to important physiological and pathophysiological mechanisms involved in adaptive and maladaptive responses to acute anemia.
Genes involved in inflammation
The dramatic up-regulation of mRNA expression of several chemokines and cytokines may promote leukocyte migration into the brain. These cytokines are often produced under pathological conditions, such as mechanical trauma, multiple sclerosis, Alzheimer’s disease, and ischemia.15–19 In addition, the dramatic increase in prostaglandin E synthase may also mediate a cerebral inflammatory response.20–22
Two other pro-inflammatory factors (CCAAT/enhancer binding protein [C/EBP β and δ]) were also up-regulated at 6 hr. The C/EBP family plays an important role in the control of cellular proliferation and differentiation.23,24 C/EBP β and δ are pivotal transcription factors involved in the regulation of genes encoding many acute phase proteins and cytokines induced after cerebral ischemia reperfusion, e.g., IL-6, IL-1β, and TNFα.23,25–27 Protein measurements demonstrating an increase in IL-6 and TNFα in the cerebral cortex of anemic rats supports this microarray finding. Astrocytes expressing C/EBP β and δ may produce these cytokines, which in turn can induce the expression of C/EBP β and δ in the surrounding cells. This feed-forward mechanism could serve to augment the inflammatory response following acute anemia.27
Finally, P-selectin at 6 hr and von Willebrand factor (vWF) were up-regulated at 24 hr in the anemic cerebral cortex. Low erythrocyte aggregation might trigger endothelium-dependent thrombogenic and inflammatory responses during acute isovolemic hemodilution.28 Moreover, serum levels of vWF may be a good indicator of cerebral endothelial injury and poor outcome following cerebral injury.29,30 Finally, our microarray data showed a significant up-regulation of S100A9, a marker of organ injury at 6 and 24 hr.31,32 However, this pro-inflammatory molecule did not increase to the same degree as the Anesthesia Control at 24 hr. Clearly, further detailed investigations are required to better understand the roles of numerous genes with novel and complex expression patterns in the cerebral cortex following acute hemodilution.
Genes involved in angiogenesis
Previous experimental studies have provided evidence of VEGF up-regulation,12,33 angiogenesis, and increased capillary density in anemic animals and humans.33–35 Our previous studies have demonstrated evidence of increased vascular endothelial growth factor (VEGF) RNA and protein in brain homogenates from anemic rats, suggesting that angiogenesis may be acutely activated. The current microarray data demonstrated that angiopoietin 2 (angiogenic factor) increased progressively after 6 and 24 hr of anemia. Up-regulation of angiopoietin 2, thrombospondin 1, and other angiogenic genes provides additional evidence that angiogenesis may have been activated in the cerebral cortex of anemic rats. Angiopoietin 2 interacts with VEGF to promote new blood vessel formation. In our study, cerebral VEGF levels were significantly elevated, but at a magnitude below our last detection filter (1.8-fold increase). The concomitant increase in VEGF and angiopoietin 2 gene expression in the anemic cerebral cortex may play a role in mediating the increase in capillary density observed during anemia and hypoxia exposure.35,36 Conversely, angiopoietin 2 up-regulation may play a maladaptive role when expressed alone by promoting apoptosis or increasing permeability of the blood brain barrier.37 In addition, there is growing evidence that brain chemokines play crucial roles in the neuro-glio-vascular interaction,38 ischemic injury and repair,39,40 and angiogenesis.41 In the current study, one angiogenic (CXCL1) and three angiostatic CXCs (CKCL9-11) were dramatically up-regulated by anemia at 6 hr but not at 24 hr. Further investigations will be required to determine the effect of this response on angiogenesis during anemia.
Genes involved in organ protection
Our microarray results showed the up-regulation of three organ protective genes, i.e., cyclin-dependent kinase inhibitor 1A (CDKN1A) and metallothionein 1a at 6 hr and annexin II at 24 hr. Annexin II plays a role in promoting glial cell survival and protects neurons and glial cells against hypoxia injury.42 Metallothioneins (MTs) are small cystein-rich proteins involved in cytoprotection during pathology. Metallothionein isoforms I and II are important for host defense responses, immunoregulation, cell survival, and brain repair.43–45 Consistent with this notion, experiments using genetically altered mice have suggested that MT-I + II are essential for wound healing and neuronal survival after brain injury secondary to trauma, hypoxia, and ischemia.46–49 The mechanisms underlying MT’s protective effect may relate to a reduction in the inflammatory response associated with central nervous system (CNS) injury.50,51 It is plausible that the profound up-regulation of MT in the cerebral cortex of anemic rats may play an anti-inflammatory neuroprotective role. We also observed a significant up-regulation of CDKN1A and lipocalin 2 (also named neutrophil gelatinase-associated lipocalin [NGAL]) in the brain of anemic rats. These molecules may be protective by facilitating DNA repair and by decreasing inflammatory response in the brain.52,53
The risk of false discovery
A number of lines of evidence suggest that many of our microarray findings reflect true biological responses. First, the microarray data that suggest a strong up-regulation of pro-inflammatory response are supported by our protein measurements (TNFα, IL-6, MCP-1). Second, the current microarray data supports previous RNA and protein data suggesting up-regulation of proteins involved in angiogenesis (VEGF, Ang. 2). Third, many of the genes that are up-regulated at 6 hr were not identified at 24 hr, while a few genes remained elevated at both 6 and 24 hr. Fourth, two control groups were utilized for the comparison to baseline. Fifth, a high degree of stringency was applied to the cut-off for changes in gene expression (greater than two-fold). However, despite these arguments, the possibility that some of our responses represent a degree of false discovery cannot be excluded.
There are several limitations to this study. We have analyzed three groups of rats and assessed changes in gene expression by microarray analysis. Although many novel pathways were identified, this method was not sensitive enough to pick up all identified changes in gene expression that have been previously demonstrated, i.e., inducible and neuronal nitric oxide synthase.8,12 In addition, this method does not assess important post-transcriptional modification RNA or protein stabilization, which have been observed in the cerebral cortex of anemic rats, i.e., Hypoxia Inducible Factor-1α.12 However, the microarray analysis did demonstrate evidence of up-regulation of novel genes that were involved with these biological mechanisms. Our protein analysis provided support for activation of inflammatory mechanisms. The lack of down-regulated genes was likely a combination of several factors: (1) The design set the cutoff at a fold change of two, which may have eliminated discovery of down-regulated genes; (2) The experimental time points may not have captured the down-regulated genes, i.e., earlier than 6 hr or later than 24 hr; (3) The sample size was too small to detect down-regulated genes. However, further experimental studies will be required to confirm the significance of these genes using mRNA (RT-PCR) and protein analysis (Western blot).
Conclusions
This study demonstrates the potential usefulness of DNA microarray technology as an approach for generating hypotheses regarding molecular pathways associated with anemia. We focused our study on genes involved in inflammation, angiogenesis, and organ protection. We have shown changes in the expression levels of numerous genes that have not been previously associated with acute hemodilution. Most of the genes have been previously identified in models of cerebral ischemia and hypoxia and cardiac ischemia reperfusion. Further studies are necessary to characterize these genes and to identify their physiological impact after anemia.
References
Weiskopf RB, Kramer JH, Viele M, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology 2000; 92: 1646–52.
Weiskopf RB, Toy P, Hopf HW, et al. Acute isovolemic anemia impairs central processing as determined by P300 latency. Clin Neurophysiol 2005; 116: 1028–32.
Jonas RA, Wypij D, Roth SJ, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg 2003; 126: 1765–74.
Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg 2003; 125: 1438–50.
Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 2002; 102: 237–44.
Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet 1988; 1: 727–9.
Homi HM, Yang H, Pearlstein RD, Grocott HP. Hemodilution during cardiopulmonary bypass increases cerebral infarct volume after middle cerebral artery occlusion in rats. Anesth Analg 2004; 99: 974–81.
McLaren AT, David MC, Zhang H, Liu E, Mok L, Hare GM. A potential role for inducible nitric oxide synthase in the cerebral response to acute hemodilution. Can J Anesth 2009; 56: 502–9.
Duebener LF, Sakamoto T, Hatsuoka S, et al. Effects of hematocrit on cerebral microcirculation and tissue oxygenation during deep hypothermic bypass. Circulation 2001; 104: I260–4.
Hare GM, Mazer CD, Mak W, et al. Hemodilutional anemia is associated with increased cerebral neuronal nitric oxide synthase gene expression. J Appl Physiol 2003; 94: 2058–67.
McClintick JN, Edenberg HJ. Effects of filtering by present call on analysis of microarray experiments. BMC Bioinformatics 2006; 7: 49.
McLaren AT, Marsden PA, Mazer CD, et al. Increased expression of HIF-1{alpha}, nNOS, and VEGF in the cerebral cortex of anemic rats. Am J Physiol Regul Integr Comp Physiol 2007; 292: R403–14.
Botchkina GI, Meistrell ME 3rd, Botchkina IL, Tracey KJ. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med 1997; 3: 765–81.
Weiss JM, Downie SA, Lyman WD, Berman JW. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood–brain barrier. J Immunol 1998; 161: 6896–903.
Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA 1999; 96: 6873–8.
Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol 2001; 2: 108–15.
Gourmala NG, Buttini M, Limonta S, Sauter A, Boddeke HW. Differential and time-dependent expression of monocyte chemoattractant protein-1 mRNA by astrocytes and macrophages in rat brain: effects of ischemia and peripheral lipopolysaccharide administration. J Neuroimmunol 1997; 74: 35–44.
Sorensen TL, Tani M, Jensen J, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest 1999; 103: 807–15.
Wang X, Yue TL, Barone FC, Feuerstein GZ. Monocyte chemoattractant protein-1 messenger RNA expression in rat ischemic cortex. Stroke 1995; 26: 661–5.
Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A. Inflammatory response: pathway across the blood–brain barrier. Nature 2001; 410: 430–1.
Engblom D, Ek M, Andersson IM, et al. Induction of microsomal prostaglandin E synthase in the rat brain endothelium and parenchyma in adjuvant-induced arthritis. J Comp Neurol 2002; 452: 205–14.
Ikeda-Matsuo Y, Ikegaya Y, Matsuki N, Uematsu S, Akira S, Sasaki Y. Microglia-specific expression of microsomal prostaglandin E2 synthase-1 contributes to lipopolysaccharide-induced prostaglandin E2 production. J Neurochem 2005; 94: 1546–58.
Cardinaux JR, Allaman I, Magistretti PJ. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia 2000; 29: 91–7.
Cortes-Canteli M, Pignatelli M, Santos A, Perez-Castillo A. CCAAT/enhancer-binding protein beta plays a regulatory role in differentiation and apoptosis of neuroblastoma cells. J Biol Chem 2002; 277: 5460–7.
Akira S, Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev 1992; 127: 25–50.
DeGraba TJ. The role of inflammation after acute stroke: utility of pursuing anti-adhesion molecule therapy. Neurology 1998; 51: S62–8.
Yan SF, Tritto I, Pinsky D, et al. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J Biol Chem 1995; 270: 11463–71.
Morariu AM, Maathuis MH, Asgeirsdottir SA, et al. Acute isovolemic hemodilution triggers proinflammatory and procoagulatory endothelial activation in vital organs: role of erythrocyte aggregation. Microcirculation 2006; 13: 397–409.
Yokota H, Naoe Y, Nakabayashi M, et al. Cerebral endothelial injury in severe head injury: the significance of measurements of serum thrombomodulin and the von Willebrand factor. J Neurotrauma 2002; 19: 1007–15.
Frijns CJ, Kasius KM, Algra A, Fijnheer R, Rinkel GJ. Endothelial cell activation markers and delayed cerebral ischaemia in patients with subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2006; 77: 863–7.
Thorey IS, Roth J, Regenbogen J, et al. The Ca2+-binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. J Biol Chem 2001; 276: 35818–25.
Yanagisawa R, Takano H, Inoue K, et al. Complementary DNA microarray analysis in acute lung injury induced by lipopolysaccharide and diesel exhaust particles. Exp Biol Med (Maywood) 2004; 229: 1081–7.
Dunst J, Becker A, Lautenschlager C, et al. Anemia and elevated systemic levels of vascular endothelial growth factor (VEGF). Strahlenther Onkol 2002; 178: 436–41.
El Hasnaoui-Saadani R, Pichon A, Marchant D, et al. Cerebral adaptations to chronic anemia in a model of erythropoietin-deficient mice exposed to hypoxia. Am J Physiol Regul Integr Comp Physiol 2009; 296: R801–11.
Rakusan K, Cicutti N, Kolar F. Effect of anemia on cardiac function, microvascular structure, and capillary hematocrit in rat hearts. Am J Physiol Heart Circ Physiol 2001; 280: H1407–14.
Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and deadaptation to prolonged mild hypoxia. J Appl Physiol 2002; 93: 1131–9.
Nag S, Papneja T, Venugopalan R, Stewart DJ. Increased angiopoietin2 expression is associated with endothelial apoptosis and blood–brain barrier breakdown. Lab Invest 2005; 85: 1189–98.
Banisadr G, Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines and brain functions. Curr Drug Targets Inflamm Allergy 2005; 4: 387–99.
Frangogiannis NG. Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res 2004; 53: 585–95.
Kocher AA, Schuster MD, Bonaros N, et al. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J Mol Cell Cardiol 2006; 40: 455–64.
Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev 2005; 16: 593–609.
Han S, Zhang KH, Lu PH, Xu XM. Effects of annexins II and V on survival of neurons and astrocytes in vitro. Acta Pharmacol Sin 2004; 25: 602–10.
Aschner M, Cherian MG, Klaassen CD, Palmiter RD, Erickson JC, Bush AI. Metallothioneins in brain—the role in physiology and pathology. Toxicol Appl Pharmacol 1997; 142: 229–42.
Chung RS, Vickers JC, Chuah MI, West AK. Metallothionein-IIA promotes initial neurite elongation and postinjury reactive neurite growth and facilitates healing after focal cortical brain injury. J Neurosci 2003; 23: 3336–42.
Penkowa M. Metallothioneins are multipurpose neuroprotectants during brain pathology. FEBS J 2006; 273: 1857–70.
Carrasco J, Penkowa M, Hadberg H, Molinero A, Hidalgo J. Enhanced seizures and hippocampal neurodegeneration following kainic acid-induced seizures in metallothionein-I + II-deficient mice. Eur J Neurosci 2000; 12: 2311–22.
Giralt M, Penkowa M, Lago N, Molinero A, Hidalgo J. Metallothionein-1 + 2 protect the CNS after a focal brain injury. Exp Neurol 2002; 173: 114–28.
Trendelenburg G, Prass K, Priller J, et al. Serial analysis of gene expression identifies metallothionein-II as major neuroprotective gene in mouse focal cerebral ischemia. J Neurosci 2002; 22: 5879–88.
van Lookeren Campagne M, Thibodeaux H, van Bruggen N, et al. Evidence for a protective role of metallothionein-1 in focal cerebral ischemia. Proc Natl Acad Sci USA 1999; 96: 12870–5.
Hidalgo J, Aschner M, Zatta P, Vasak M. Roles of the metallothionein family of proteins in the central nervous system. Brain Res Bull 2001; 55: 133–45.
Penkowa M, Tio L, Giralt M, et al. Specificity and divergence in the neurobiologic effects of different metallothioneins after brain injury. J Neurosci Res 2006; 83: 974–84.
Zaman K, Ryu H, Hall D, et al. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci 1999; 19: 9821–30.
MacManus JP, Graber T, Luebbert C, et al. Translation-state analysis of gene expression in mouse brain after focal ischemia. J Cereb Blood Flow Metab 2004; 24: 657–67.
Acknowledgment
Supported by the Canadian Anesthesiologists’ Society and the Department of Anesthesia, St. Michael’s Hospital. Dr. Hare is a recipient of the Bristol-Myers Squibb-CAS Career Scientist Award. The authors wish to acknowledge the technical support of Elaine Liu and Rong Qu.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Briet, F., Mazer, C.D., Tsui, A.K.Y. et al. Cerebral cortical gene expression in acutely anemic rats: a microarray analysis. Can J Anesth/J Can Anesth 56, 921–934 (2009). https://doi.org/10.1007/s12630-009-9201-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-009-9201-z