Abstract
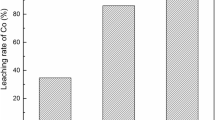
To effectively separate and recover Co(II) from the leachate of spent lithium-ion battery cathodes, we investigated solvent extraction with quaternary ammonium salt N263 in the sodium nitrite system. NO −2 combines with Co(II) to form an anion [Co(NO2)3]−, and it is then extracted by N263. The extraction of Co(II) is related to the concentration of NO −2 . The extraction efficiency of Co(II) reaches the maximum of 99.16%, while the extraction efficiencies of Ni(II), Mn(II), and Li(I) are 9.27%–9.80% under the following conditions: 30vol% of N263 and 15vol% of iso-propyl alcohol in sulfonated kerosene, the volume ratio of the aqueous-to-organic phase is 2:1, the extraction time is 30 min, and 1 M sodium nitrite in 0.1 M HNO3. The theoretical stages require for the Co(II) extraction are performed in the McCabe—Thiele diagram, and the extraction efficiency of Co(II) reaches more than 99.00% after three-stage counter-current extraction with Co(II) concentration of 2544 mg/L. When the HCl concentration is 1.5 M, the volume ratio of the aqueous-to-organic phase is 1:1, the back-extraction efficiency of Co(II) achieves 91.41%. After five extraction and back-extraction cycles, the Co(II) extraction efficiency can still reach 93.89%. The Co(II) extraction efficiency in the actual leaching solution reaches 100%.
Similar content being viewed by others
References
E. Peek, T. Åkre, and E. Asselin, Technical and business considerations of cobalt hydrometallurgy, JOM, 61(2009), No. 10, p. 43.
Q. Dehaine, L.T. Tijsseling, H.J. Glass, T. Törmänen, and A.R. Butcher, Geometallurgy of cobalt ores: A review, Miner. Eng., 160(2021), art. No. 106656.
M. Chandra, D.W. Yu, Q.H. Tian, and X.Y. Guo, Recovery of cobalt from secondary resources: A comprehensive review, Miner. Process. Extr. Metall. Rev., 43(2022), 6, p. 679.
B. Wassink, D. Dreisinger, and J. Howard, Solvent extraction separation of zinc and cadmium from nickel and cobalt using Aliquat 336, a strong base anion exchanger, in the chloride and thiocyanate forms, Hydrometallurgy, 57(2000), No. 3, p. 235.
R. Golmohammadzadeh, F. Faraji, and F. Rashchi, Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review, Resour. Conserv. Recycl., 136(2018), p. 418.
M.K. Tran, M.T.F. Rodrigues, K. Kato, G. Babu, and P.M. Ajayan, Deep eutectic solvents for cathode recycling of Li-ion batteries, Nat. Energy, 4(2019), No. 4, p. 339.
W.Y. Wang, C.H. Yen, and J.K. Hsu, Selective recovery of cobalt from the cathode materials of NMC type Li-ion battery by ultrasound-assisted acid leaching and microemulsion extraction, Sep. Sci. Technol., 55(2020), No. 16, p. 3028.
X.H. Zheng, Z.W. Zhu, X. Lin, et al., A mini-review on metal recycling from spent lithium ion batteries, Engineering, 4(2018), No. 3, p. 361.
X.L. Zeng, J.H. Li, and N. Singh, Recycling of spent lithium-ion battery: A critical review, Crit. Rev. Environ. Sci. Technol., 44(2014), No. 10, p. 1129.
F. Gu, J.F. Guo, X. Yao, P.A. Summers, S.D. Widijatmoko, and P. Hall, An investigation of the current status of recycling spent lithium-ion batteries from consumer electronics in China, J. Clean. Prod., 161(2017), p. 765.
L. Sun and K.Q. Qiu, Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries, Waste Manage., 32(2012), No. 8, p. 1575.
A.M. Bernardes, D.C.R. Espinosa, and J.A.S. Tenório, Recycling of batteries: A review of current processes and technologies, J. Power Sources, 130(2004), No. 1–2, p. 291.
L. Li, J. Ge, R.J. Chen, F. Wu, S. Chen, and X.X. Zhang, Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries, Waste Manage., 30(2010), No. 12, p. 2615.
J.Q. Xu, H.R. Thomas, R.W. Francis, K.R. Lum, J.W. Wang, and B. Liang, A review of processes and technologies for the recycling of lithium-ion secondary batteries, J. Power Sources, 177(2008), No. 2, p. 512.
L. Chen, X.C. Tang, Y. Zhang, L.X. Li, Z.W. Zeng, and Y. Zhang, Process for the recovery of cobalt oxalate from spent lithium-ion batteries, Hydrometallurgy, 108(2011), No. 1–2, p. 80.
M.B.J.G. Freitas, V.G. Celante, and M.K. Pietre, Electrochemical recovery of cobalt and copper from spent Li-ion batteries as multilayer deposits, J. Power Sources, 195(2010), No. 10, p. 3309.
J.G. Kang, G. Senanayake, J. Sohn, and S.M. Shin, Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272, Hydrometallurgy, 100(2010), No. 3–4, p. 168.
H.Y. Wang, K. Huang, Y. Zhang, et al., Recovery of lithium, nickel, and cobalt from spent lithium-ion battery powders by selective ammonia leaching and an adsorption separation system, ACS Sustainable Chem. Eng., 5(2017), No. 12, p. 11489.
X.P. Chen, Y.B. Chen, T. Zhou, D.P. Liu, H. Hu, and S.Y. Fan, Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries, Waste Manage., 38(2015), p. 349.
N. Ocaña and F.J. Alguacil, Cobalt—manganese separation: The extraction of cobalt(II) from manganese sulphate solutions by cyanex 301, J. Chem. Technol. Biotechnol., 73(1998), No. 3, p. 211.
B. Pośpiech and W. Walkowiak, Separation of copper(II), co-balt(II) and nickel(II) from chloride solutions by polymer inclusion membranes, Sep. Purif. Technol., 57(2007), No. 3, p. 461.
A.H. Blitz-Raith, R. Paimin, R.W. Cattrall, and S.D. Kolev, Separation of cobalt(II) from nickel(II) by solid-phase extraction into Aliquat 336 chloride immobilized in poly(vinyl chloride), Talanta, 71(2007), No. 1, p. 419.
H.C. Kao and R.S. Juang, Kinetic analysis of non-dispersive solvent extraction of concentrated Co(II) from chloride solutions with Aliquat 336: Significance of the knowledge of reaction equilibrium, J. Membr. Sci., 264(2005), No. 1–2, p. 104.
M. Majdan, J. Mierzwa, and P. Sadowski, On the separation of Co and Ni from chloride media with Aliquat 336-TBP and Aliquat 336-TOPO, Monatsh. Chem., 128(1997), No. 2, p. 113.
N.A. Milevskii, I.V. Zinov’eva, Y.A. Zakhodyaeva, and A.A. Voshkin, Separation of Li(I), Co(II), Ni(II), Mn(II), and Fe(III) from hydrochloric acid solution using a menthol-based hydrophobic deep eutectic solvent, Hydrometallurgy, 207(2022), art. No. 105777.
Y.Z. Wei, T. Arai, M. Kumagai, and Q.M. Feng, Adsorption behavior of various metal ions in nitrite medium and separation of some metals by anion exchange, J. Ion Exch., 14(2003), Suppl., p. 305.
H. Chen, S. Gu, Y.X. Guo, et al., Leaching of cathode materials from spent lithium-ion batteries by using a mixture of ascorbic acid and HNO3, Hydrometallurgy, 205(2021), art. No. 105746.
J.R. Ju, Y.L. Feng, H.R. Li, et al., Separation of Cu, Co, Ni and Mn from acid leaching solution of ocean cobalt-rich crust using precipitation with Na2S and solvent extraction with N235, Korean J. Chem. Eng., 39(2022), No. 3, p. 706.
Y.J. Yang, Y.N. Yang, C.L. He, et al., The adsorption and desorption behavior and mechanism research of cobalt, nickel and copper in nitrite—sulfuric acid system, Sep. Sci. Technol., 57(2022), No. 12, p. 1848.
Z.S. Liu, J. Huang, Y.M. Zhang, et al., Separation and recovery of vanadium and aluminum from oxalic acid leachate of shale by solvent extraction with Aliquat 336, Sep. Purif. Technol., 249(2020), art. No. 116867.
K. Wang, G.Q. Zhang, M.Z. Luo, and M. Zeng, Separation of Co and Mn from acetic acid leaching solution of spent lithiumion battery by Cyanex272, J. Environ. Chem. Eng., 10(2022), No. 5, art. No. 108250.
A.A. Nayl, M.M. Hamed, and S.E. Rizk, Selective extraction and separation of metal values from leach liquor of mixed spent Li-ion batteries, J. Taiwan Inst. Chem. Eng., 55(2015), p. 119.
H.E. Rizk, Y.A. El-Nadi, and N.E. El-Hefny, Extractive separation of scandium from strongly alkaline solution by quaternary ammonium salt, Solvent Extr. Ion Exch., 38(2020), No. 3, p. 350.
H. Zhang, C.M. Li, X.J. Chen, et al., Layered ammonium vanadate nanobelt as efficient adsorbents for removal of Sr2+ and Cs+ from contaminated water, J. Colloid Interface Sci., 615(2022), p. 110.
J.J. Meng, C.L. He, Y.J. Li, et al., Enhanced adsorption and separation of gallium using silica-based P507-TBP/SiO2-P adsorbent from sulfuric acid solution, Microporous Mesoporous Mater., 314(2021), art. No. 110859.
L.Z. Jiao, D.M. Dong, W.G. Zheng, et al., Determination of nitrite using UV absorption spectra based on multiple linear regression, Asian J. Chem., 25(2013), No. 4, p. 2273.
H. Benalia and D. Barkat, Solvent extraction studies of cobalt(II) by capric acid from sodium sulfate solution, J. Dispersion Sci. Technol., 38(2017), No. 9, p. 1247.
J. Cañón and A.V. Teplyakov, XPS characterization of cobalt impregnated SiO 2 and γ-Al2O3, Surf. Interface Anal., 53(2021), No. 5, p. 475.
G. Kowalski, J. Pielichowski, and M. Grzesik, Characteristics of polyaniline cobalt supported catalysts for epoxidation reactions, Sci. World J., 2014(2014), art. No. 648949.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 51804084), the Natural Science Foundation of Guangxi Province, China (No. 2021GXNSFAA220096), and the Science and Technology Major Project of Guangxi Province, China (No. AA172 04100).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
The authors declare no potential conflict of interest.
Supplementary Information
12613_2022_2571_MOESM1_ESM.docx
Solvent extraction and separation of cobalt from leachate of spent lithium-ion battery cathodes with N263 in nitrite media
Rights and permissions
About this article
Cite this article
Yang, Y., Yang, Y., He, C. et al. Solvent extraction and separation of cobalt from leachate of spent lithium-ion battery cathodes with N263 in nitrite media. Int J Miner Metall Mater 30, 897–907 (2023). https://doi.org/10.1007/s12613-022-2571-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-022-2571-8